Translate this page into:
Respiration And Heart Rate Variability : A Review With Special Reference To Its Application In Aerospace Medicine
Abstract
Heart Rate Variability (HRV) indices provide a non-invasive assessment of cardiovascular control mechanisms. The last few years have witnessed a burgeoning growth of research effort and literature on various HRV indices, encompassing a large cross section of cardiovascular and autonomic pliysiology/psychophysiology. The analysis finds varied applications in a multitude of fields including Aerospace Medicine. After presenting a brief summary of linear and nonlinear HRV indices, the present article reviews the effects of various respiratory influences on different HRV estimates with the mechanisms involved therein. Certain examples are given, from the field of Aerospace Medicine, of the application of HRV analysis wherein respiration could be a potential confounder. Concerns expressed regarding effects of controlling the respiratory variables on HRV indices are addressed and, finally, the issue of susceptibility of non-linear HRV estimates to breathing is dealt with.
Keywords
Heart rate variability
Spectral analysis
Sympatho-vagal balance
Entropy
In the recent past, there has been a spurt of research efforts involving heart rate variability (HRV) which provides a non-invasive assessment of cardiovascular control mechanisms. A search, made in PubMed, returns more than 4,000 citations. Both linear and nonlinear HRV measures have been used. Mostly, the HRV is measured using linear estimates which include various time and frequency domain indices. However, a few non-linear indices of HRV have also been proposed.
Linear measures of HRV
Linear measures of HRV include various time and frequency domain indices. Time domain indices provide information on total variability over a period of time (without resolving it further).
The time domain indices could be derived from direct measurements of the RR intervals (or instantaneous heart rate) or from the differences between RR intervals. The former include SDNN (standard deviation of the normal to normal interval ie, the square root of variance), SDANN (standard deviation of the average NN interval calculated over short periods, usually 5 min and is an estimate of the changes in heart rate due to cycles longer than 5 min) and the SDNN index, the mean of the 5-min standard deviation of the NN interval calculated over 24 hours measuring the variability due to cycles shorter than 5 min. The most commonly used measures derived from interval differences include RMSSD, the square root of the mean squared differences of successive NN intervals, NN50, the number of interval differences of successive NN intervals greater than 50 ms, and pNN50, the proportion derived by dividing NN50 by the total number of NN intervals [1].
Frequency domain indices provide information on both total variability as well as its distribution as a function of frequency. Various spectral methods are available. Spectral analysis of RR intervals derived from short term recordings of 2 to 5 min yields three separate bands -
A very low frequency (VLF) band located in the less than 0.04 Hz (with dubious physiologic significance).
A low frequency (LF) band located in the 0.04-0.15 Hz range (which derives from short term regulation of blood pressure).
A high frequency (HF) band with a very large range from 0.15-0.50 Hz (reflecting momentary respiratory influences on the heart rate or respiratory sinus arrhythmia) [1].
The HF component is decreased by tilting or parasympathetic blocking drugs and is increased by sympathetic blocking drugs [2,3]. Therefore, the HF component is thought to provide a quantitative and specific index of vagal cardiac function. On the other hand, the LF component is increased while standing and the increase is blocked by intravenous propranolol [2,3]. Moreover, this component is not found in quadriplegic humans who have severe dysfunction of the sympathetic nervous system [4]. Therefore, the LF component in humans has been interpreted as an indicator mainly of sympathetic influence. Consequently, the LF/HF ratio is considered to be a convenient index of sympatho-vagal interaction.
Nevertheless, interpretation of LF components is controversial. It is considered by some (vide supra) as a marker of sympathetic modulation (especially when expressed in normalised units) and by others as a parameter that includes both sympathetic and parasympathetic influences [5].
Nonlinear measures of HRV
There is some evidence for the involvement of nonlinear phenomena in the genesis of HRV. It is conceived that assessment of HRV with nonlinear measures may supply information different from and additional to that derived through linear measures. Different approaches have been employed for the nonlinear analysis of HRV. In nonlinear dynamics theory, the so-called state space is reconstructed from sequences of heartbeat periods which are generally defined as the time duration between successive R waves. Subsequently, the state space and the dynamic behaviour of the reconstructed dynamics can be quantified (eg, with measures of dimension or Lyapunov exponents).
Since evolution in time of HRV signal shows self similarity properties, certain methods are based on fractal analysis. These include- 1/f scaling of Fourier spectra, Hurst exponent ‘H’ and Detrended Fluctuation Aanalysis (DFA). In 1/f scaling, the slope ß of the power spectrum is calculated by a regression analysis of log(power) and log(frequency) plots of the smoothed power spectrum usually over the frequency range of 10-2 to 10-4 Hz. Hurst exponent is a measure of the smoothness of fractal time series based on the asymptotic behaviour of the rescaled range of the process. DFA of heart rate variability was initially presented as a specialised time-domain technique, in which the series of RR intervals undergoes cumulative summing and then segmentation into short segments. Within each segment, the degree of dispersion of the cumulated time series away from its linear trend is measured (as the sum of squares of residuals after subtracting the linear regression line). The total of the squared residuals for the individual segments is calculated for the overall data set. The entire process is then repeated with a different segment length. As the segments become longer, the degree of dispersion away from the linear regression lines within the segments tends to increase. The rate at which this total dispersion increases as the segments become longer is measured as a slope (a) on a log-log’plot over particular regions of segment length, eg, 4–16 beats or 16–64 beats. As a matter of fact, it is the range used, originally, by Ho et al [6]. Steeper slopes are said to show higher complexity.
The above three indices are related to each other as follows -
Another approach to nonlinear measures of HRV is the quantification of complexity from the point of view of information theory. The sequence of heart periods can be analyzed with the help of entropy measures such as Shannon entropy or renormalized entropy. These are often used in conjunction with the concept of symbolic dynamics or coding theory, ie, reducing the amount of information by transforming the original time series into a symbolic sequence with a small set of symbols. The concept of entropy, as it applies to signals like R-R intervals, is to quantify the repetition of patterns in that signal. Larger values of entropy correspond to greater apparent randomness or irregularity, whereas smaller values correspond to more instances of recognizable patterns in the data. Another entropy measure for quantification of regularity in a time series is the approximate entropy (ApEn). ApEn(m, r, n) is approximately equal to the negative average natural logarithm of the conditional probability that two sequences that are similar for ‘m’ data points remain similar within a tolerance ‘r’ at the next point and sample entropy. However, ApEn has significant weaknesses, notably its strong dependence on sequence length and its poor self-consistency and certain alternatives have been suggested [7].
For data representation, Poincarè sections, low-dimension attractor plots, singular value decomposition, and attractor trajectories have been used [1].
In the field of aerospace medicine, HRV analysis has been employed to study such diverse stressors as hyporbaric hypoxia, both micro and hypergravity and vibration. It has also been used in a number of fields in psychophysiology, as well. For example, HRV measures have been investigated extensively as indices of mental workload.
Despite such a varied and wide application of HRV, not much attention has been paid, by the researchers, to control or factor out the confounding effects of respiration on HRV indices. Brown and his associates [8], in their review of studies reporting human R-R interval power spectra, observed that only 51 % of the studies controlled respiratory rate, 11 % controlled tidal volume, and 11 % controlled both respiratory rate and tidal volume. In a more recent review, Schipke et al [9] found that respiration was referenced in approximately 15% of the papers on heart rate variability returned from a search in the Index Medicus. These observations are ironically surprising because the respiratory influences on HRV are so protean and powerful that no worthwhile interpretation can be made of the analysis of HRV unless these respiratory confounders are controlled. A variety of respiratory influences can affect HRV.
In the present article, a review is made of effect of various respiratory influences on different HRV estimates with the mechanisms involved. Certain examples are given, from the field of Aerospace Medicine, of the application of HRV analysis wherein respiration could be a potential confounder. Concerns expressed regarding effects of controlling the respiratory variables on HRV indices are addressed in the subsequent section. Since all the above issues refer almost exclusively to the linear estimates, a separate account is given of the iusceptibility of nonlinear HRV estimates to breathing.
What All Respiratory Parameters Could Affect The HRV Estimates?
The respiratory parameters which can affect HRV estimates, include-respiratory frequency (Rf) [9,10], tidal volume [10], end tidal partial pressure of carbon di-oxide (PETco2) [10,11], the time ratio of expiration/inspiration [12] and respiratory dead space [13]. Since breathing through an oro-nasal mask or mouthpiece can also affect breathing pattern and components of ventilatory responses to chemostimuli [14], it can be extrapolated that it will also influence HRV estimates-an observation of importance in the aerospace settings.
How Do The Above Respiratory Parameters Affect HRV?
Respiratory frequency & tidal volume
The variation of heart rate in the frequency range of respiration, known as respiratory sinus arrhythmia (RSA), was already described by Ludwig in 1847 [15]. Despite many past studies, the precise mechanisms of respiration-induced SA are still debated. The theories which have been proposed are not mutually exclusive. The most important ones are the modulation of cardiac filling pressure by respiratory movements [16], the direct respiratory modulation of parasympathetic and sympathetic neural activity in the brain stem [17] and the respiratory modulation of the baroreceptor feedback control [18].
In a recent review of published evidence, Eckberg [19] summarised that respiratory fluctuations of muscle sympathetic nerve activity and electrocardiographic R-R intervals result primarily from the action of a central ‘gate’ that opens during expiration and closes during inspiration. Parallel respiratory fluctuations of arterial pressures and R-R intervals are thought to be secondary to arterial baroreflex physiology-changes in systolic pressure provoke changes in the R-R interval. However, growing evidence suggests that these parallel oscillations result from the influence of respiration on sympathetic and vagal-cardiac motoneurones rather than from baroreflex physiology.
In yet another synthesis, RSA could be a physiologic phenomenon reflecting respiratory-circulatory interactions improving the efficiency of pulmonary gas exchange. The matched timing of alveolar ventilation and its perfusion with RSA within each respiratory cycle could save energy expenditure by suppressing unnecessary heartbeats during expiration and ineffective ventilation during the ebb of perfusion (vide infra).
Change in end tidal PCO2
The most likely mechanism responsible for increased RSA magnitude, with an increase in PETco2, is chemostimulation that enhances respiratory modulation of vagal outflow. Stimulation of carotid chemoreceptors by increased arterial Pco2 has primarily an excitatory effect on vagal preganglionic neurons to the heart in the expiratory phase [20,21]. In unanesthetized trained dogs, Yasuma and Hayano [22] reported that hypercapnia (PETco2 up to 54 mmHg) increases RSA magnitude by 62% with no concomitant changes in mean R-R interval.
Increased RSA could also be a manifestation of cardiorespiratory interactions [23] which contribute to CO2 elimination by reducing physiological dead space and intrapulmonary shunt, ie, matching the distribution of pulmonary blood flow to lung volume during each respiratory cycle [24]. An increased demand for CO, elimination may therefore enhance RSA to facilitate pulmonary gas exchange. Regulation of RSA magnitude by PaCO2 could complement CO2-modulated changes in airway smooth muscle tone in controlling dead space. Hypercapnia is shown to both decrease tracheal diameter in anesthetized dogs [25] and causes bronchoconstriction in decerebrate cats [26]. In anesthetized dogs, Hayano et al [24] also showed that RSA reduces physiological dead space, ie, the alveolar dead space, by matching perfusion to ventilation during each respiratory cycle.
It has been shown in conscious humans [27] that increase in RSA magnitude due to the direct effects of CO2 are independent of changes in tidal volume and breathing frequency.
Relative timing of inspiration and expiration
Strauss-Blasche et al [12] showed that RSA can also be modulated by a third respiratory variable. In their experiment, examining the effect of a variation in inspiration and expiration times on heart rate variability, the subjects were given 2 x two min trials of controlled breathing with either short inspiration followed by long expiration or long inspiration followed by short expiration. Average expiration/inspiration time ratios were 1.0 and 3.4, respectively and the respiration rate in both trials was approximately 10 cycles/min. In trials with short inspiration followed by long expiration, RSA (measured by mean absolute differences and by the high frequency band) was significantly larger than in trials with long inspiration followed by short expiration. This effect could not be accounted for by differences in respiratory rate or amplitude. The higher RSA during fast/slow respiration is primarily due to a more pronounced phasic heart rate increase during inspiration, indicating that inspiratory vagal blockade is sensitive to the steepness of inspiration.
Cardiac aliasing
There is yet another mechanism reported to be involved in mediating respiratory fluctuations of heart beat. Witte et al [28] observed that if a special relationship exists between mean heart rate (fHR) and mean frequency of breathing (fB) such that fB is greater than 1/2 fHR, RSA can be observed in a frequency range which is lower than the frequency of breathing. The mathematical fundamentals of this physiological phenomenon are the same as those for the ‘aliasing’ effect in signal sampling. The authors termed it ‘cardiac aliasing’ and could experimentally demonstrate it in adult rabbits and dogs as well as in human neonates.
Respiratory dead space
Hirsch JA and Bishop B [14] demonstrated that choice of a mouthpiece or a face mask can differentially change breathing pattern and all the components of ventilatory responses to chemostimuli. These breathing apparatus effects did not appear to be a simple consequence of a shift from oronasal to oral breathing. In an interesting study, Furutani Y [13] evaluated the effect of the dead space induced by the face mask used in the expiratory gas exchange analysis on the measurement of heart rate variability using ECG records for 5 min during spontaneous respiration under the conditions of supine rest, sitting on the bicycle ergometer with and without a face mask. The value of LF/HF increased from supine rest to sitting in accordance to the change of body position, but the value of LF/HF when sitting with the face mask decreased to the level during supine rest. The value of HF/TP decreased from supine rest to sitting, but when sitting with the face mask returned to that during supine rest. It was also seen that the value of LF/HF decreased from supine rest to sitting with the face mask in the smaller tidal volume group (tidal volume<570 ml) and there was a significant correlation between the change of the value of LF from supine rest to sitting with the face mask and the tidal volume. These results suggest that the power spectrum of heart rate variability is strongly influenced by the dead space induced by the face mask used in expiratory gas exchange analysis. Even though the sympathetic activation from supine rest to sitting in subjects with the smaller tidal volume is unclear, interpretation of the results of heart rate variability with or without the face mask used requires care.
Is Respiratory Influence Confined To Only High (Respiratory) Frequencies In The HRV Power Spectrum?
Schipke et al [9] examined the effect of controlled respiration at six different breathing frequencies on HRV indices derived from short term recordings of six minutes each. Breathing frequencies ranged from below the low-frequency range (LF) of the power spectrum (0.03 Hz) to above HF (0.50 Hz). Heart rate remained unchanged throughout the protocol, indicating a steady haemodynamic state. HRV differed up to 33% in SDNN, 37% in RMSSD and 75% in pNN50 between the different respiration rates. LF power differed up to 72%, HF power up to 36% and R up to 48%. These results show that respiration can change HRV power spectra both in high and low frequency regions (Figure-1, drawn from the data presented by Schipke et al, refers).
![BREATHING FREQUENCY & SPECTRAL HRV POWER [Drawn from the data of Schipke et al, 1999]](/content/110/2004/48/1/img/IJASM-48-064-g001.png)
BREATHING FREQUENCY & SPECTRAL HRV POWER
[Drawn from the data of Schipke et al, 1999]
Novak [29] studied the dynamics of the respiratory and cardiovascular systems by continuously slowing respiration from 0.46 to 0.05 Hz. During rest, the nonrespiratory-to-respiratory frequency ratios were not affected by occasional slow breathing. As respiration slowed to 0.07-0.09 Hz, the frequency content of the respiration and cardiovascular variables increased sharply and nonlinearly to a maximum that exceeded values at higher frequencies. The nonrespiratory frequency content remained stable in the 0.01-to 0.05-Hz range and did not significantly differ from that at rest. In contrast, the 0.05- to 0.1-Hz component was suppressed. A slow 0.012- to 0.017-Hz rhythm modulated respiration and hemodynamic fluctuations at both respiratory and nonrespiratory frequencies. The study indicated that respiration input should be considered in the interpretation of global spectra.
However, recently, independence of low-frequency rhythms from respiratory activity is reported [30].
Sasano [27] failed to observe any change in low frequency power or LF/HF ratio over a range of PETco2 (30,40 & 50 mm Hg) in conscious human subjects despite a significant increase in RS A. Mean R-R interval did not differ at PETco2 of 40 and 50 mmHg but was less at 30 mmHg and changes in tidal volume and breathing frequency were prevented.
Even if low frequency region of HRV power spectra is relatively insusceptible from the respiratory influences, the latter will affect interpretation of spectral values in low frequency region in normalised terms due to a significant change in the total power.
Certain Examples From Aerospace Settings Wherein HRV Estimates Could Be Confounded From Respiratory Influences
Hypoxia
Assessment of autonomic function during exposures to hypoxia is important as the former may affect tolerance to this stress and could contribute to certain specific syndromes viz, acute mountain sickness, high altitude pulmonary edema, and high altitude cerebral edema. HRV analysis has generally shown an increase in cardiac sympathetic activity after acute exposure to hypoxia [31, 32, 33] without a significant change in the fractal component (which indicated overall ‘irregularity’ of HRV). Certain studies have, however, reported results which are not in consonance with the above observations. For example, Sevre et al [34] have shown a transient reduction in both parasympathetic and sympathetic activity during stepwise exposure to high altitude. Pre-adaptation to hypoxia is shown to modulate HRV responses in rats [35] but not in humans [31]; this difference could be due to difference in the period of acclimatisation. HRV analysis has also been used to demonstrate ethnic variations in reactions to hypoxia [36] and assessment of baroreflex responsiveness in hypoxia [34]. In almost all the above studies, the results are not without confounding effects of one or more respiratory variables (viz breathing frequency, tidal volume, PETco2, dead space etc) which were neither controlled nor monitored. All these respiratory attributes are known to change during hypoxia [37,38].
Hyperfoaria with/without hyperoxia
Bradycardia has been observed in animals and humans upon exposure to various hyperbaric environments. Because of the complexity of the hyperbaric environment, the cause of the bradycardia is not obvious. Certain investigators [39, 40] have concluded that hyperoxia is the most important variable in the development of hyperbaric bradycardia as these studies failed to observe bradycardia under normoxic conditions in unanesthetized rats despite similar density, pressure, and inert gas components.
A decrease in the resting muscle sympathetic nerve activity (MSNA) is observed in human volunteers exposed to hyperbaric conditions [41]. Normobaric hyperoxia (100% O2 at sea level) also lowers heart rate and MSNA at rest [42,43].
These observations lend support to hypothesis that hyperoxia attenuates sympathetic nerve activity. However, bradycardia in normobaric hyperoxia remains unaffected in dogs by ß-adrenoceptor blockade but is completely prevented by cholinergic blockade [44], it is prevented by intramuscular administration of atropine [45]. These observation suggest that bradycardia in normobaric hyperoxia could be mediated through parasympathetics.
To further complicate the matter, substantial degrees of bradycardia have also been observed in humans during hyperbaric exposure with normoxic or near-normoxic gas mixtures [46]. The non-O2-dependent bradycardia, thus, must be caused by other factors, such as the increased hydrostatic pressure, the increased gas density, or the increased partial pressure of metabolicaliy inert gas(es) alone or in combination. Other contributing factors could be the thermal conductivity of the ambient gas and of the breathing gas, especially if these gases include helium (He) [47].
Therefore, a multitude of efforts have been made, using analysis of HRV, to explore the precise behaviour of and interplay between sympathetic and parasympathetic branches of ANS in hyperbaric hyperoxia. These studies have yielded conflicting results [48-51]. One possible reason for the conflicting results from the above studies could be the confounding effect of respiratory variables which were neither controlled nor monitored. Other confounding variables could have been hypoventilation and carbon di-oxide retention [46] which are often reported during hyperbaric exposure. Moreover, most of the above studies used professional divers as subjects with reduced adrenergic and stress response to CO2 [52].
Tilt table & LBNP studies
A progressive decrease in end tidal PETco2 may occur during tilt due to relative hyperventilation. Whether control of breathing will help arrive at a different interpretation about autonomic function is controversial. Certain investigators have used a controlled breathing protocol during such studies [53]. On the other hand, some studies have shown that both physiologic reactions and outcome of a tilt table study may be significantly affected by paced breathing [54]. Others [55] observed that it may be possible to arrive at similar interpretation about autonomic function with and without using control of respiratory rate. Figure-2, derived from the data of this study, refers.
[Drawn from data of Patwardhan et al, 2001]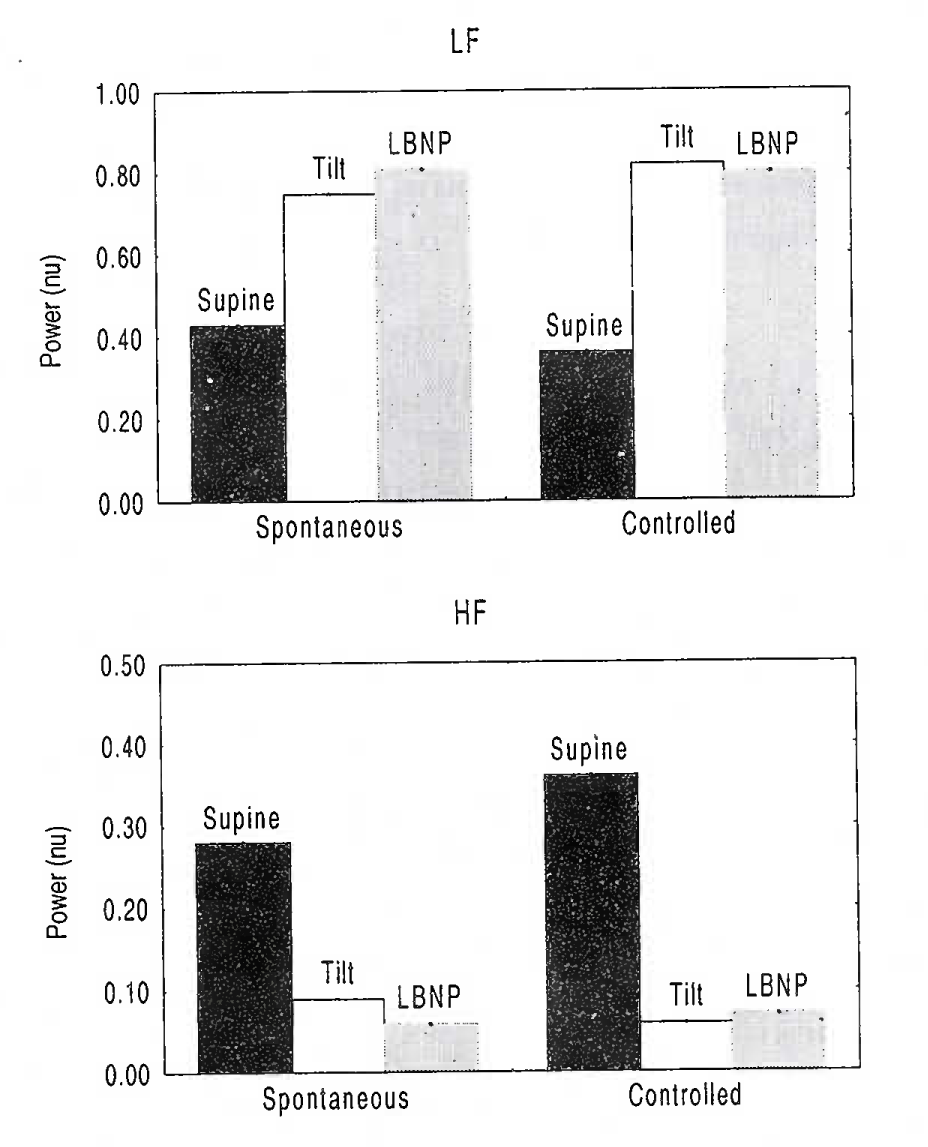
Effect Of Spontaneous & Controlled Breathing On HRV Spectral Power During Tilt and LBNP
[Drawn from data of Patwardhan et al, 2001]
However, these results are to be viewed carefully. Authors only conclude that metronomic breathing may not provide any additional insight into autonomic function than what can be obtained during spontaneous breathing. The effect could simply have been because the breathing frequency did not change much.
Hypergravic simulations
McKenzie [56] investigated the effect of simulated increases in gravity (G) force on blood pressure and heart rate variability in seven normal healthy subjects using a man-carrying centrifuge. Subjects were exposed to 3.6 Gz forces while breathing at a fixed rate and depth. Increases in G force produced increases in spectral power of systolic blood pressure and diastolic blood pressure at the respiratory frequency (0.2 Hz) and less conspicuous but significant increases in spectral power at lower frequencies (0.045-0.15 Hz). The spectral power of beat-to-beat interval did not change. Author postulated that the reduction in central blood volume produced by increased gravity is affecting blood pressure control in a similar way to that seen in hypovolaemic animals. The marked increase in blood pressure fluctuations induced by respiration at the higher G levels was viewed as a result of the alteration in venous return to the right atrium, ultimately reflected as fluctuating left ventricular output and pressure.
Contrary to this, Pipraiya [57] observed a significant reduction in total (0.04-0.40 Hz) as well as HF (0.15-0.40 Hz) power in absolute terms during centrifugation of human subjects at +3Gz for 1 minute. Change (an increase) in LF (0.04-0.15 Hz) power was found to be significant only when normalised to total power. Additionally, he observed a leftward shift of the ‘peak power frequency’ (ie, the frequency at which maximum power was concentrated) in the LF band signifying a change in the responsiveness of the sympathetic effector organs. Breathing could not be controlled but was monitored during centrifugation. The author reasoned that a change in breathing rate from 15.7±1.3 in resting sitting to 17.6±1.2 during centrifuge run was too small to explain the changes in spectral power.
Assessment of baroreflex sensitivity
HRV is also employed for the non invasive assessment of baroreflex sensitivity (BRS). Low-frequency (LF) blood pressure variability (BPV) to HRV transfer-index is a common method for this. However, this derivation assumes that all LF-HRV is caused by baroreflex feedback of LF-BPV. Nevertheless, respiration may also cause HRV by mechanisms not involving the baroreflex. Application of narrow-band (controlled) high-frequency breathing would keep such non-baroreflex-mediated HRV best out of the LF band. On the other hand, spontaneous breathing, because of its broad-band character, might cause extra, non-baroreflex-mediated, HRV in the LF band, while paced LF breathing would even concentrate most non-baroreflex-mediated HRV in the LF band. In an interesting study, Frederiks et al [58] have demonstrated the possibility of a significant overestimation of BRS when respiration was not controlled and/or was not of high frequency.
WhoLebodj ribration
Author could get only one abstract in MEDLINE on the application of HRV analysis in whole body vibration. In this study [59], HRV, in conjunction with subjective indices, has been used to characterise the effect of different vibration frequencies on fatigue during simulated driving. The study could display differential effect of two frequencies (1.8 and 6 Hz) of whole body sinusoidal vibration in the vertical axis (0.05 G) on sympatho-vagal balance discerned as LF/HF ratio. Apparently, the respiratory parameters were not controlled/monitored. Such vibration is reported to cause true hyperventilation due to alarm, stimulation of stretch receptors in the lungs or superimposition of oscillatory airflow on respiratory excursions [60]. It is likely that variation in respiratory effects during exposure to the two different frequencies might have contributed to the observed difference in LF/HF ratio.
Psychophysiologie studies
HRV indices have variously been used in psychophysiological studies. In the field of aerospace medicine, the indices have been used with impunity for the assessment of human mental workload. These respiratory effects may induce changes in HRV indices in an opposite direction. HRV indices are being tried also as indicators of arousal [61] & fatigue [62] and for characterisation of personality [63], In most of the studies, none of the respiratory variables have been controlled or monitored. Respiratory pattern is demonstrated to change with an increase in difficulty [64]. Subjects breath slowly and deeply under mental load [65].
Microgravity
HRV has been used, during and after exposure to microgravity or its simulation, as a non invasive tool to monitor autonomic functions [66-71]. It has also been used to assess the efficacy of pharmacological intervention in preventing the effects of increase in the effects of Epinephrine induced by simulated microgravity [72] and to characterise the effect of geomagnetic fluctuations on human body in space [68]. Ventilatory parameters have not been controlled/monitored in all these studies. Microgravity results into an increase in respiratory, reduction in tidal volume and dead space. End tidal PCO2 may also increase due to spacecraft atmospheric conditions [73].
Will Controlling Breathing Itself Influence HRV?
Please also refer to section on ‘Tilt table studies & LBNP’. There has been a concern that controlling breathing itself may change HRV due to cortical involvement and confound the results. Patwardhan et al [74] reported, in their first study, that controlled and spontaneous breathing did not differ with regard to vagal control. He attributed the changes observed in his study in HF power during metronomic breathing to ‘respiratory sinus arrhythmia versus breathing frequency relationship’ reported by Hirsch & Bishop [75] who have reported that, for breathing frequencies greater than 0.1 Hz, respiratory sinus arrythmia amplitude (in beats/min) decreased at the rate of approximately 20 dB/decade of breathing frequency. Hirsch & Bishop [75] also showed that ‘respiratory sinus arrhythmia versus breathing frequency relationship’ was the same during both spontaneous and metronomic breathing. With the use of this information, Patwardhan et al [74] calculated that for a 0.097-decade increase in breathing frequency (ie, from the mean spontaneous breathing frequency of 0.24 Hz to the metronomic breathing frequency of 0.30 Hz) respiratory arrhythmia should drop by 1.25 beats/min or the oscillation in RR interval should decrease by about 21 ms. In their study, power in high frequency decreased from 1932 to 733 ms2 ie, the oscillatory RR interval amplitude decreased from 62 to 38 ms, a change (24 ms). consistent with that predicted using the results of Hirsch.
However, in their second study, Patwardhan and his associates [76] reported that override of spontaneous respiratory pattern generator reduced HF power.
Pagani et al [2] observed increase in heart rate and HF power during controlled respiration. This observation is surprising in view of pacing frequency being higher than the mean breathing frequency of the subjects.
Results of Stark et al [77] further complicate the issue. The authors examined no change in any of the spectral components of HRV while comparing spontaneous breathing condition with a frequency matched paced condition. This was despite a significant increase in heart rate with an increase in breathing frequency in both paced and unpaced conditions.
Are Nonlinear HRV Measures Not Susceptibile To Respiratory Influences?
Certain investigators [78] have reported that the quantitative geometrical analysis of short-term RR interval variability from the Poincare plots (and also the time domain measure RMSSD) were not significantly affected by changes in the breathing rate. Thus, these indices may be more suitable for the measurement of cardiac vagal outflow during the ‘free-running’ ambulatory conditions wherein it is not possible to measure/control the ventilatory parameters. Kanters et al [79] have shown that nonlinear dynamics in heart rate variability is not a nonlinear input from the respiration into the cardiovascular oscillator. The authors did not find nonlinear dynamics (measured as the correlation dimension and the nonlinear prediction error) to differ significantly during spontaneous versus forced respiration conditions. Occasionally, insusceptibility of nonlinear HRV indices to breathing frequency and depth has been quoted as a justification for not controlling or monitoring the breathing [50].
However, there are studies which have shown susceptibility of even nonlinear indices to ventilatory variables [80-81]. The requirement of controlling or monitoring respiratory variables is expressed and failure to control / monitor breathing is accepted as one of the limitations in the study [82].
It is to be appreciated that the physiological origin for these nonlinearities is unknown. From the mathematical point of view, spectral measures resemble scaling indices when analyzed as normalized units during strictly controlled external conditions, because both describe relative changes in the characteristics of HR fluctuations over different time scales rather than the magnitude of HRV. Certain studies have established the correspondence between nonlinear and linear indices of HRV. For example, Francis et al [83] have shown that the al and a 2 indices derived from, Detrended Fluctuation Analysis, are simply frequency-weighted versions of the spectral ratios LF/(HF + LF) and VLF/(LF + VLF), respectively. Similarly, Brennan et al [84] have shown that SD1 & SD2 of Poincaré plot are related to time domain HRV measures as follows –
This further enacts exercise of caution in the interpretation of nonlinear HRV estimates.
Conclusions And Recommendations
It should be clear from the foregoing review that a multitude of respiratory influences can affect various HRV indices and may confound the results unless controlled. Even though interpretation of HRV indices derived from ‘free-running’ experiments with spontaneous breathing could, sometimes, remain unaffected in qualitative terms, it is always better and sometimes essential to control/factor out all such possible confounders.
References
- Heart rate Variability-Standards of measurement, physiological interpretation and clinical use. Task Force-Europcan Society of Cardiology and The North American Society of Pacing and Electrophysiology. Eur Heart J. 1996;17:354-81.
- [Google Scholar]
- Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympatho-vagal interaction in man and conscious dog. Circ Res. 1986;59:178-93.
- [Google Scholar]
- Assessment of autonomic function in humans by heart rate spectral analysis. Am J Physiol Heart Circ Physiol. 1985;248:H151-3.
- [Google Scholar]
- Power spectral analysis of heart rate variability in traumatic quadriplegic. Am J Physiol Heart Circ Physiol. 1990;258:H1722-6.
- [Google Scholar]
- Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat-to-beat cardiovascular control. Science. 1981;213:220-2.
- [Google Scholar]
- Predicting survival in heart failure case and control subjects by use of fully automated methods. Circulation. 1997;96:842-8.
- [Google Scholar]
- Physiological time-series analysis using approximate entropy and sample entropy. Am J Physiol Heart Circ Physiol. 2000;278:H2039-49.
- [Google Scholar]
- Important influence of respiration on human R-R interval power spectra is largely ignored. J Appi Physiol. 1993;75:2310-17.
- [Google Scholar]
- Effect of respiration rate on short-term heart rate variability. J Clin Basic Cardiol. 1999;2:92-4.
- [Google Scholar]
- The effect of carbon dioxide, respiratory rate and tidal volume on human heart rate variability. Acta Anaesthesiol Scand. 2004;48:93-101.
- [Google Scholar]
- Interactions between CO2 chemoreflexes and arterial baroreflexes. Am J Physiol Heart Circ Physiol. 1998;274:H2177-87.
- [Google Scholar]
- Relative timing of inspiration and expiration affects respiratory sinus Arrhythmia. Clin Exp Pharmacol Physiol. 2000;27:601-6.
- [Google Scholar]
- Influence of the dead space induced by the face mask on the measure of heart rate variability. J Cardiol. 1997;29:171-6.
- [Google Scholar]
- Human breathing patterns on mouthpiece or face mask during air, CO2 or low O2 . J Appi Physiol. 1982;53:1281-90.
- [Google Scholar]
- Beiträge zur Kenntniss des Einflusses der Respirationsbewegung auf den Blutlauf im Aortensystem. Arch Anat Physiol. 1847;13:242-302.
- [Google Scholar]
- Synaptic mechanisms involved in the inspiratory modulation of vagal cardio-inhibitory neurons in the cat. J Physiol. 1984;365:65-78.
- [Google Scholar]
- Phase relationship between normal human respiration and baroreflex responsiveness. J Physiol. 1980;304:489-502.
- [Google Scholar]
- The effects of stimulation of the carotid body chemoreceptors on heart rate in the dog. J Physiol. 1958;144:148-66.
- [Google Scholar]
- Peripheral chemoreceptors and cardiovascular regulation. Physiol Rev. 1994;74:543-94.
- [Google Scholar]
- Augmentation of respiratory sinus arrhythmia in response to progressive hypercapnia in conscious dogs. Am J Physiol Heart Circ Physiol. 2001;280:H2336-41.
- [Google Scholar]
- Central control of the cardiovascular and respiratory systems and their interactions in vertebrates. Physiol Rev. 1999;79:855-916.
- [Google Scholar]
- Respiratory sinus arrhythmia: a phenomenon improving pulmonary gas exchange and circulatory efficiency. Circulation. 1996;94:842-47.
- [Google Scholar]
- PCO2 affects tracheal tone during apnea in anesthetized dogs. J Appi Physiol. 1996;81:1184-9.
- [Google Scholar]
- Bronchomotor responses to hypoxia and hypercapnia in decerebrate cats. J Appi Physiol. 1995;78:117-23.
- [Google Scholar]
- Direct effect of Paco, on respiratory sinus arrhythmia in conscious humans. Am J Physiol Heart Circ Physiol. 2002;282:H973-6.
- [Google Scholar]
- Evidence of a previously undescribed form of respiratory sinus arrhythmia (RSA)- the physiological manifestation of ‘cardiac aliasing’. Pflügers Arch. 1988;412:442-4.
- [Google Scholar]
- Influence of respiration on heart rate and blood pressure fluctuations. J Appi Physiol. 1993;74:617-26.
- [Google Scholar]
- Respiratory modulation of human autonomic rhythms. Am J Physiol Heart Circ Physiol. 2001;280:H2674-88.
- [Google Scholar]
- Effects of high altitude acclimatization on heart rate variability in resting humans. Eur J Appi Physiol Occup Physiol. 1996;73:521-8.
- [Google Scholar]
- Effects of acute exposure to simulated altitude on heart rate variability during exercise. J Appi Physiol. 1996;81:1223-9.
- [Google Scholar]
- Analysis of heart rate variability during acute exposure to hypoxia. Space Med Med Eng (Beijing). 2001;14:328-31.
- [Google Scholar]
- Reduced autonomic activity during stepwise exposure to high altitude. Acta Physiol Scand. 2001;173:409-17.
- [Google Scholar]
- Heart rate variability in rats acclimatized to high altitude. High Alt Med Biol. 2003;4:375-87.
- [Google Scholar]
- Reserved higher vagal tone under acute hypoxia in Tibetan adolescents with long-term migration to sea level. Jpn J Physiol. 2002;52:51-6.
- [Google Scholar]
- Control of breathing at high altitude In: Hornbein TF, Schoene RB, eds. High Altitude: An exploration of human adaptation. New York: Marcel Dekker Inc; 2001. p. :139-73. In:
- [Google Scholar]
- Mechanics of breathing In: Hornbein TF, Schoene RB, eds. High Altitude : An exploration of human adaptation. New York: Marcel Dekker Inc; 2001. p. :175-98. In:
- [Google Scholar]
- Contribution of environmental factors in development of hyperbaric bradycardia. J Appi Physiol. 1981;50:4731-5.
- [Google Scholar]
- Cardiac output changes during hyperbaric hyperoxia. Int Arch Occup Environ Health. 2001;74:119-22.
- [Google Scholar]
- Sympathetic nervous and hemodynamic responses to lower body negative pressure in hyperbaria in men. Am J Physiol Regul Integr Comp Physiol. 2002;282:R38-45.
- [Google Scholar]
- Hyperoxia lowers sympathetic activity at rest but not during exercise in humans. Am J Physiol Regul Integr Comp Physiol. 1991;260:R873-8.
- [Google Scholar]
- influence of age on the sympathetic neural adjustments to alterations in systemic oxygen levels in humans. Am J Physiol Regulatory Integrative Comp Physio. 1997;273:R690-5.
- [Google Scholar]
- Response time, autonomic mediation, and reversibility of hyperoxic bradycardia in conscious dogs. J Appi Physiol. 1993;74:634-42.
- [Google Scholar]
- Effects of oxygen breathing on the heart rate, blood pressure and cardiac index of normal men-resting, with reactive hyperaemia and after atropine. J Clin invest. 1962;41:126-32.
- [Google Scholar]
- Mixed-gas saturation diving In: Fregly MJ, Blatteis CM, eds. Handbook of Physiology. Section-4: Environmental Physiology, Vol-II, Chapter-44. New York: Oxford University Press; 1996. p. :1023-48. 1996 In:
- [Google Scholar]
- Hyperbaric bradycardia and hypoventilation in exercising men: effects of ambient pressure and breathing gas. J Appl Physiol. 1999;87:1428-32.
- [Google Scholar]
- Autonomic Nervous System in the Patients with Coronary Artery Diseases during Hyperbaric Oxygenation Therapy. 1st Virtual congress in Cardiology Sep 1, 1999 to Ma 31, Mar 2000 Available from URL: http://pcvc.sminter.com.ar/cvirtual/tlibres/tnn2324/tnn2324.htm
- [Google Scholar]
- Hyperbaric oxygen increases parasympathetic activity in professional divers. Acta Anaesthesiol Scand. 2000;170:39-44.
- [Google Scholar]
- Instantaneous beat-to-beat variability reflects vagal tone during hyperbaric hyperoxia. Undersea Hyperb Med. 2003;30:29-36.
- [Google Scholar]
- Autonomie mechanisms of bradycardia during nitrox exposure at 3 Atmospheres Absolute in humans. Aviat Space Environ Med. 2003;74:643-8.
- [Google Scholar]
- Hyperbaria-breath hold dives In: Fregly MJ, Blatteis CM, eds. Handbook of Physiology. New York: Oxford University Press; 1996. p. :979-95. 1996 In: Section-4: Environmental Physiology, Vol-II, Chapt-42
- [Google Scholar]
- Heart rate and blood pressure variabilities during graded head-up tilt. J Appi Physiol. 1995;78:212-6.
- [Google Scholar]
- Paced breathing can prevent vasovagal syncope during head-up tilt testing. Can J Cardiol. 2003;19:698-700.
- [Google Scholar]
- Heart rate variability during sympatho-excitatory challenges: comparison between spontaneous and metronomic breathing. Integr Physiol Behav Set. 2001;36:109-20.
- [Google Scholar]
- Heart rate and blood pressure variability in subjects exposed to simulated increases in gravity. Exp Physiol. 1993;78:825-34.
- [Google Scholar]
- Study of the effects of +Gz acceleration on autonomic control of heart rate and rhythm by the time domain and power spectral analysis. MD Thesis submitted to Rajiv Gandhi University of Health Sciences 2004. Institute of Aerospace Medicine, Bangalore (INDIA)
- The importance of high-frequency paced breathing in spectral baroreflex sensitivity assessment. J Hypertens. 2000;18:1635-44.
- [Google Scholar]
- Effect of different vibration frequencies on heart rate variability and driving fatigue in healthy drivers. Int Arch Occup Environ Health. 2004;77:205-12.
- [Google Scholar]
- Vibration In: Ernsting J, Nicholson AN, Rainford DJ, eds. Aviation Medicine (3 ed). Oxford, UK: Butterworth-Heinemann; 1999. p. :177-91. In: Chapter-13
- [Google Scholar]
- Sleep position, autonomic function, and arousal. Arch Dis Child Fetal Neonatal Ed. 1998;78:F189-94.
- [Google Scholar]
- Spectral analysis of heart rate variability as an indicator of driver fatigue. Ergonomics. 1982;25:663-72.
- [Google Scholar]
- Power spectral analysis of heart rate variability in Type As and Type Bs during mental workload. Psychosom Med. 1992;54:462-70.
- [Google Scholar]
- Respiration in psychophysiology: Methods and applications. Biol Psychol. 1992;34:179-204.
- [Google Scholar]
- Physiological indices of workload in a simulated flight task. Biol Psychol. 1996;42:323-42.
- [Google Scholar]
- Sympatho-vagal responses in humans to thermoneutral head-out water immersion. Aviat Space Environ Med. 1997;68:1109-14.
- [Google Scholar]
- Autonomic regulation of circulation and cardiac contractility during a 14-month space flight. Acta Astronaut. 1998;42:159-73.
- [Google Scholar]
- Regulation of autonomic nervous system in space and magnetic storms. Adv Space Res. 1998;22:227-34.
- [Google Scholar]
- Parasympathetic activity during parabolic flight, effect of LBNP during microgravity. Aviat Space Environ Med. 2001;72:361-7.
- [Google Scholar]
- Parasympathetic heart rate modulation during parabolic flights. Eur J Appi Physiol. 2003;90:83-91.
- [Google Scholar]
- Microgravity alters respiratory sinus arrhythmia and short-term heart rate variability in humans. Am J Physiol Heart Circ Physiol. 2003;284:H1995-2006.
- [Google Scholar]
- Yohimbine administration prevents over-responsiveness to epinephrine induced by simulated microgravity. Aviat Space Environ Med. 2000;73:735-42.
- [Google Scholar]
- Pulmonary gas exchange and its determinants during sustained microgravity on Spacelabs SLS-1 and SLS-2. J Appi Physiol. 1995;79:1290-8.
- [Google Scholar]
- Voluntary control of breathing does not alter vagal modulation of heart rate. J App Physiol. 1995;78:2087-94.
- [Google Scholar]
- Respiratory sinus arrhythmia in humans: how breathing pattern modulates heart rate. Am J Physiol Heart Circ Physiol. 1981;241:H620-9.
- [Google Scholar]
- Override of spontaneous respiratory pattern generator reduces cardiovascular parasympathetic influence. J App Physiol. 1995;79:1048-54.
- [Google Scholar]
- Effects of paced respiration on heart period and heart period variability. Psychophysiology. 2000;37:302-9.
- [Google Scholar]
- Time domain, geometrical and frequency domain analysis of cardiac vagal outflow: effects of various respiratory patterns. Clin Physiol. 2001;21:365-76.
- [Google Scholar]
- Influence of forced respiration on nonlinear dynamics in heart rate variability. Am J Physiol Regulatory Integrative Comp Physio. 1997;272:R1149-54.
- [Google Scholar]
- Respiratory influences on non-linear dynamics of heart rate variability in humans. Biol Cybem. 1997;77:1-10.
- [Google Scholar]
- Nonlinear measures of heart rate time series: influence of posture and controlled breathing. Auton Neurosci. 2000;83:148-58.
- [Google Scholar]
- Cardiac interbeat interval dynamics from childhood to senescence-comparison of conventional and new measures based on fractals and chaos theory. Circulation. 1999;100:393-9.
- [Google Scholar]
- Physiological basis of fractal complexity properties of heart rate variability in man. J Physiol. 2002;542:619-29.
- [Google Scholar]
- Do Existing Measures of Poincaré Plot Geometry Reflect Nonlinear Features of Heart Rate Variability? IEEE Trans Biomed Eng. 2001;48:1342-7.
- [Google Scholar]






