Translate this page into:
Assessment of hypoxia tolerance to high-altitude exposure following sleep deprivation

-
Received: ,
Accepted: ,
How to cite this article: Bharadhwaj S, Mohapatra SS, Sarkar R, Ningaiah M, Raju AV. Assessment of hypoxia tolerance to high-altitude exposure following sleep deprivation. Indian J Aerosp Med 2022;66:2-8.
Abstract
Objectives:
The adverse effects of hypoxia and sleep deprivation (SD) have been studied separately but there is little literature on their effects when acting in conjunction. Hence, the effects of SD on hypoxia tolerance and cognitive performance when exposed to altitude were investigated.
Material and Methods:
In a cross-sectional repetitive measure design, 15 healthy volunteers assessed on cognitive performance were made to perform cognitive tests (Dual Task Test, Stroop Test [ST], and Digit Symbol Substitution Test [DSST] of Psychometric Evaluation Designed for Aviators test battery) on the ground and at a simulated altitude of 17,000 ft in a hypobaric chamber on 4 days. On the 2nd and 3rd day, the tests were done following 2 h of SD the previous night, and on the 4th day, the same was repeated following normal recovery sleep. Heart rate (HR) and respiratory rate (RR) were measured on the ground and 17,000 ft. The Lake Louise Acute Mountain Sickness (LLAMS) scores were used to assess the severity of symptoms of hypoxia and time to arterial oxygen desaturation of 75% was used to measure hypoxia tolerance.
Results:
Although not statistically significant, the LLAMS scores showed an increasing trend on SD days with an improvement in recovery of sleep. No statistically significant differences in the HR (F = 0.569, P = 0.637) and RR (F = 0.569, P = 0.637) were observed across the days of SD or recovery. The time to oxygen desaturation to 75% also showed no significant effect (F = 0.587, P = 0.625). A statistically significant increase in response times of two cognitive tests (ST and DSST) was observed on exposure to altitude on all 4 days. The three cognitive tests did not show any statistically significant effect following 2 h of SD or recovery.
Conclusion:
The study could not elicit a statistically significant effect of 2 h of SD across 2 consecutive nights on the cognitive performance measures on exposure to hypoxia.
Keywords
Hypoxia
Hypoxia tolerance
Sleep deprivation
Cognition
Cognitive tests
Response time
INTRODUCTION
Hypoxia is an important aviation stressor in high-altitude flying operations. This is especially the case in helicopter crew who fly maintenance sorties in unpressurized helicopters for transport of men and materials between forwarding posts for replenishment, removal, and evacuation.[1] Psychological functions such as mental, psychomotor, and cognitive performances are impaired on exposure to reduced oxygen tension at altitude and are of great operational and practical significance in the aviation environment.
Performance of well-learned and practiced tasks is generally preserved adequately up to an altitude of at least 10,000 ft, but when alveolar oxygen tension falls to below 38–40 mmHg (i.e., above an altitude of 16,000–18,000 ft), psychomotor and cognitive functions get impaired significantly. Simple reaction time, pursuit tasks, choice reaction time, tasks involving complex eye-hand coordination, muscular coordination, conceptual reasoning, and short- and long-term memory have been found to get impaired under hypoxia.[1,2]
Sleep is a basic physiological requirement for optimum cognitive and psychomotor functioning of individuals. Sleep deprivation (SD) has been found to cause impairment in alertness, attention, vigilance, and performance of tasks.[3] Taking this into consideration, we can logically assume that SD would have a compounding influence on the effects of hypoxia, causing greater concern for aerospace safety. Although these two issues have been studied separately,[2-4] there is little if any, literature available regarding the interplay between both the factors. This is relevant in the scenario of operations in the Armed Forces, as the aircrew is likely to be exposed to both hypoxia and short spells of SD. It, thus, appears that hypoxia tolerance may well be affected by lack of sleep. Keeping this as a background, a need was felt to study the combined effects of hypoxia and short spells of SD to see if they have any additive effect on performance, compromising aerospace safety.
The study aimed to assess hypoxia tolerance to high-altitude exposure following SD. The objectives of the study were to determine SD in individuals based on sleep-wake data through actigraphy and sleep diary, monitor and compare various physiological parameters such as heart rate (HR), respiratory rate (RR), and oxygen saturation (SpO2) recorded before and after the exposure to simulated hypobaric hypoxia, assess the symptoms of hypoxia by employing Lake Louise AMS Score, determine the time-lapse for achieving SpO2 of 75%, and objectively assess the cognitive performance impairment in the hypobaric hypoxic condition among the sleep-deprived personnel using a cognitive testing tool Psychometric Evaluation Designed for Aviators (pSuMEDhA).
MATERIAL AND METHODS
Subjects
Fifteen healthy male (13) and female (12) volunteers with a mean age of 33.53 ± 3.6 years were included in the study. The subjects were asked not to consume alcohol for 12 h and coffee/tea/smoking for 2 h before the test. Individuals with a history of any comorbidity, sleep disorders, respiratory illness, claustrophobia, and those not adhering to study protocol were excluded from the study. Clearance was obtained from the Institute Ethics Committee and written informed consent was obtained from all participants.
Materials
The following materials were used.
Explosive decompression chamber (EDC)
The EDC simulator located in the Department of High-Altitude Physiology and Hyperbaric Medicine, Institute of Aerospace Medicine (IAM), Bengaluru, was used to simulate hypobaric hypoxia equivalent to 17,000 ft.
Actigraphy device
Philips Respironics Actiwatch was used to monitor the sleep pattern of the subjects in this study. It was a wrist-worn activity data recorder that can record data relevant to circadian rhythms and sleep parameters in any instance where quantifiable analysis of physical motion is desirable. Each participant was asked to wear the configured Actiwatch at least for 4 days for data collection.
Finger pulse oximeter
A finger pulse oximeter was used to monitor the SpO2 of the subjects during the simulation at 17,000 ft altitude. The LED display in the pulse oximeter helped to monitor the SpO2 and time to oxygen saturation of 75%.
pSuMEDhA
This is a PC-based neurocognitive tool developed at IAM. Three cognitive tests, namely, the dual-task test (DTT), Stroop test (ST), and digit symbol substitution test (DSST) from pSuMEDhA, were selected for this study as they covered the domains of psychomotor function, executive function, and working memory. Moreover, within the time of useful consciousness (TUC) available at 17,000 ft, only these three relevant tasks could be undertaken. DTT consists of two simultaneous tasks. In the first task, a yellow ball will move around the screen and a change in color of the ball to RED will require the participant to react with a keypress. In addition to this task, the participant will also be required to track the yellow ball with a mouse. Stroop Test is presented in two phases. The first phase consists of matching the word appearing in the box with the same-colored square presented below. In the second phase, the ink color, in which the word is presented, is matched to the same-colored square presented below. The subject is supposed to read each word as quickly as possible and respond. This evaluates the reading ability concerning the color-word interference effect. Digit Symbol Substitution Test consists of a set of nonsense symbols, along with digits from 1 to 9 placed as a legend on top of the screen. One random nonsense symbol appears on the screen and the participant must identify the corresponding number from the legend and press that numerical key as quickly as possible. In case of quick responses, the next random symbols will keep appearing for the entire duration of 5 min.
Study design
The study was designed as a cross-sectional observational study with a within-subject repeated-measure study design. The main hypothesis of the study was that there would be a deterioration effect of “2 h sleep deprivation (SD)” on hypoxia tolerance (time to SpO2 of 75%) and cognitive performance on acute exposure to 17,000 feet altitude.
Experimental protocol
The protocol involved testing three pSuMEDhA tests on day 1 with optimal sleep, day 2 with 2 h of SD, day 3 with 2 h of SD, and day 4 with a return to normal sleep, under conditions of simulated hypobaric hypoxia of 17,000 feet. The tests would be conducted on 4 days for each subject. Testing on day 1 was done after the subjects had maintained good sleep hygiene and slept well with their optimal sleep level. An Ear Clearance Run was given by exposing the subject to 10,000 feet in the EDC with an ascent and descent rate of 3000 ft/min to screen against possible barotrauma. Their baseline physiological parameters of HR and RR on the ground and at a simulated altitude of 17,000 ft were recorded. The time for desaturation of arterial blood to 75% was recorded at 17,000 ft. Subjective symptoms of hypoxic exposure were scored on the Lake Louise Acute Mountain Sickness (LLAMS) scale. On the night of the 1st day, the sleep time of the subjects was delayed by 2 h, thus subjecting them to 2 h of SD. On the next day morning (day 2), the same protocol was followed like day 1. Following the day 2 experimentation, subjects were again delayed to bed by 2 h. The following day (day 3), the testing, thus, assessed the effects of partial SD of 2 h and cumulative sleep loss of 4 h. On day 3 night, the subjects were advised to have normal recovery sleep, and the assessment was repeated on the following day (day 4). Physiological parameters of HR, RR, time to SpO2 of 75%, and LLAMS scores were recorded on all the subsequent 3 days similar to baseline data collection. Four days’ sleep history was collected from the subjects to understand the average sleeping hours of the subject. Response times of DTT, ST, and DSST under experimental conditions described above along with time to achieve SpO2 of 75% and LLAMS scores at 17,000 ft of subjects formed the primary data. The physiological parameters of HR and RR on the ground and at an altitude of 17,000 ft were compared over the 4 days.
Statistical analysis
The physiological parameters of HR and RR were analyzed using a one-way analysis of variance (ANOVA) comparing the differences of means between ground and altitude of 17,000 ft. The mean LLAMS scores for subjective symptoms over 4 days were analyzed using a one-way ANOVA. The cognitive tests of DTT, ST, and DSST were analyzed using a multivariate analysis of variance (MANOVA) to look for the effects of altitude on cognition and the effects of SD on cognition.
RESULTS
LLAMS scale scores
The LLAMS scores on days 1, 2, 3, and 4 were 0.6 ± 0.91, 1.6 ± 1.18, 2.13 ± 1.60, and 1.13±1.51, respectively. The McNemar test was applied to compare the subjective symptom scores of day 1 with respective scores on the SD days and post-recovery of sleep. The LLAMS scores showed a trend of increasing on the SD days with a decline in recovery. This effect, however, was found to be statistically insignificant [Table 1].
| Day 1 and day 2 | Day 1 and day 3 | Day 1 and day 4 | |
|---|---|---|---|
| Exact sig.* (two tailed) | 0.500 | 0.063 | 1.000 |
Heart rate and respiratory rate
The HR and RR on each of the days on the ground and at 17,000 ft altitude are shown in [Table 2]. The effect of hypoxic stress was determined by the difference in means of the HR at the ground and at altitude, and these were compared for the 4 days. A one-way ANOVA on this difference of means on the 4 days showed no statistically significant variation for HR (F = 0.0175, P = 0.996) and RR (F = 0.569, P = 0.637).
| Day | D1 | D2 | D3 | D4 | ||||
|---|---|---|---|---|---|---|---|---|
| Altitude | Ground | 17K | Ground | 17K | Ground | 17K | Ground | 17K |
| Time to 75% SPO2(min) | ||||||||
| M | 13.45 | 13.24 | 12.14 | 12.71 | ||||
| SD | 2.98 | 2.63 | 3.18 | 2.99 | ||||
| DTT (ms) | ||||||||
| M | 545.40 | 580.07 | 560.73 | 585.53 | 576.53 | 586.07 | 524.53 | 559.33 |
| SD | 40.673 | 74.329 | 64.30 | 89.038 | 46.319 | 52.644 | 159.518 | 75.828 |
| ST (ms) | ||||||||
| M | −30.2 | 19 | −8.87 | 46.4 | 18 | 29.53 | −1.13 | 13.6 |
| SD | 64.302 | 43.042 | 46.696 | 85.773 | 94.913 | 79.037 | 70.833 | 58.435 |
| DSST (ms) | ||||||||
| M | 1497.80 | 1517.33 | 1346.33 | 1482.73 | 1362.53 | 1522.20 | 1388.33 | 1467.13 |
| SD | 195.789 | 182.123 | 199.467 | 269.447 | 190.810 | 227.618 | 145.073 | 140.745 |
| HR (beats/min) | ||||||||
| M | 80.46 | 100 | 80.33 | 99.8 | 82.2 | 101.86 | 81.86 | 101.13 |
| SD | 5.61 | 8.34 | 4.56 | 7.75 | 5.60 | 7.85 | 5.35 | 6.35 |
| RR (breaths/min) | ||||||||
| M | 16.66 | 28.13 | 16.73 | 29.26 | 16.8 | 29.2 | 16.6 | 28.13 |
| SD | 1.63 | 3.31 | 1.66 | 3.71 | 1.26 | 2.73 | 1.40 | 2.69 |
M: Mean, SD: Standard deviation, DTT: Dual-task test, ST: Stroop test, DSST: Digit symbol substitution test, HR: Heart rate, RR: Respiratory rate, SPO2: Oxygen saturation
Time to arterial desaturation to 75% SpO2
On exposure to the altitude of 17,000 ft, a gradual fall of SpO2 was observed on all 4 days. The values of time to desaturation to 75% SpO2 are displayed in [Table 2]. ANOVA revealed that the time to 75% SpO2 was lesser on day 2 (13.24 min) and day 3 (12.14 min) in comparison to a baseline value of day 1 (13.45 min) which again showed a rising trend on day 4 (12.71 s) [Figure 1]. However, these changes in the means were not statistically significant (F = 0.587, P = 0.625).

- The levels of mean time to 75% SpO2 on exposure to simulated hypobaric hypoxia on days 1–4 in EDC (n=15).
Dual task test
When compared with baseline ground level, the DTT reaction time showed an increase on exposure to a simulated altitude of 17,000 ft, as shown in [Figure 2]. The “Main Effects (MEs)” of altitude and day were not significant (F = 2.92, P =0.09 with observed power = 0.395 for altitude and F = 1.256, P =0.293 with observed power = 0.328 for day). The “Interaction Effect” of “Altitude_Day” on DTT reaction time was also not significant (P = 0.927).
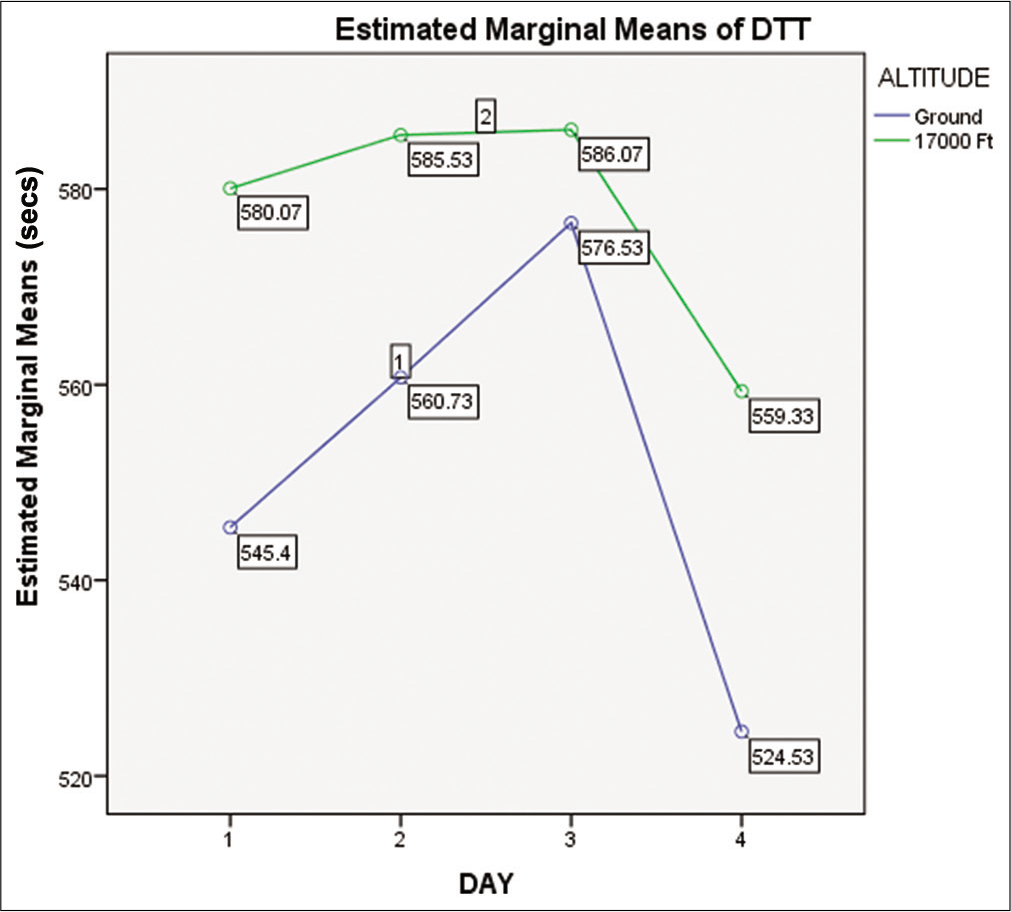
- Means of DTT time on ground and at 17,000 ft altitude on day 1, day 2, day 3, and day 4.
Stroop test
The difference between the Stroop response time of the two phases is a sensitive measure of the response inhibition in the executive functioning cognitive ability. This difference is called Stroop time (ST). When compared with the baseline value, the ST showed an increase on exposure to a simulated altitude of 17,000 ft, as shown in [Figure 3]. The “Main Effects (MEs)” of altitude were significant (F = 6.538, P = 0.012 with observed power = 0.717). However, it was not significant for day (F = 1.064, P = 0.368 with observed power = 0.282). Similarly, the “Interaction Effect” of “Altitude_Day” for ST was also not significant (P = 0.501).

- Means of Stroop time on ground and at 17,000 ft altitude on day 1, day 2, day 3, and day 4 (n = 15).
Digit symbol substitution test
When compared with the baseline value at ground level, the DSST response time revealed an increase in exposure to a simulated altitude of 17,000 ft, as shown in [Figure 4]. The “MEs” of altitude were significant (F = 7.455, P = 0.007 with power = 0.772). However, it was not significant for day (F = 1.306, P = 0.276 with power = 0.341). Similarly, the “Interaction Effect” of “Altitude_Day” for DSST time was also not significant (P = 0.552).
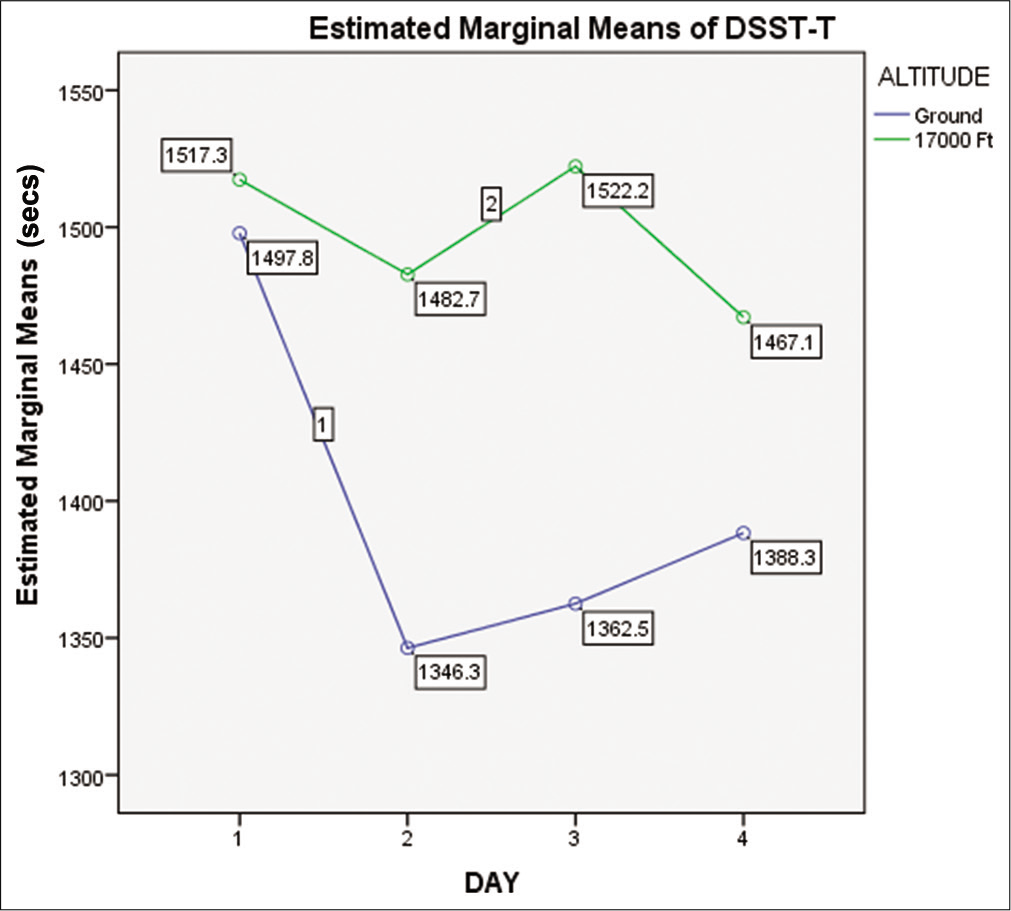
- Means of DSST time on ground and at 17,000 ft altitude on day 1, day 2, day 3, and day 4 (n=15).
The above results confirmed that there were statistically significant changes in the Stroop and DSST time on exposure to altitude. However, there were no statistically significant changes in the ST and DSST time on the sleep-deprived days (day 2 and day 3) in comparison to the normal sleep days (day 1 and day 4). The result also indicated that there is no accentuation of the effect of hypoxia on DTT, ST, and DSST due to 2 h of SD.
The results of MANOVA with altitude (ground level vs. 17,000 feet) and day (with day 1 and day 4 following normal sleep and day 2 and day 3 following 2 h of SD) and the reaction time in the three tests (DTT, ST, and DSST) are depicted in [Table 3].
| Main effects | Interaction effects (Altitude_Day) Power | Validity | |||||
|---|---|---|---|---|---|---|---|
| Day | Altitude | ||||||
| F | P | F | P | F | P | R2 | |
| DTT | 1.256 | 0.293 | 2.92 | 0.09 | 0.154 | 0.927 | 0.060 |
| ST | 1.064 | 0.368 | 6.538 | 0.012* | 0.792 | 0.501 | 0.098 |
| DSST | 1.306 | 0.276 | 7.455 | 0.007* | 0.754 | 0.522 | 0.109 |
ANOVA: Analysis of variance, DTT: Dual task test, DSST: Digit symbol substitution test, ST: Stroop test
DISCUSSION
In isolation, both the stressors, hypoxia, and SD are known to affect mental performance. In a repeated measure design, the present study intended to evaluate the combined effects of these two stressors across 4 days with 2 consecutive days of 2 h of SD and one night of recovery sleep following that. The functional neuroimaging techniques (fMRI) have shown that the regional, but not global, reduction of the cerebral blood flow, especially in frontal areas of the brain on hypoxia exposure.[4-6] Similarly, fMRI signals in the dorsolateral prefrontal cortex and intraparietal sulcus while performing attentional tasks were reduced and seen to be a reliable consequence of SD.[7-9] The literature available on the combined effect of hypoxia and SD[10,11] is scanty and gives little information on their effect on hypoxia tolerance of an individual. The present study was undertaken to see if the stress of partial SD contributes further in impairing cognition due to hypoxic hypoxia and if it has any bearing on an individual’s overall ability to tolerate hypoxia exposure. This study is thus more relevant in the setting of the Indian Armed Forces, especially during operational requirements, wherein aircrew may suffer short-term SD[12] and maybe exposed to hypoxic conditions. In the study, 2 h of SD was considered as the average sleep time of aircrew was seen to be about 6 h against a total sleep requirement of 8 h.[12-14] The present study aimed to find if tolerance to hypoxia of a subject was affected by acute partial SD of 2 h for 2 consecutive days. The effect of recovery of sleep following short spells of SD was also studied. The hypoxia tolerance on exposure to 17,000 ft simulated altitude was assessed by evaluating the scores of subjective symptoms of hypoxia, comparison of physiological parameters (HR and RR), rate of desaturation of arterial blood, and cognitive performance scores on 4 days.
The mean LLAMS score (subjective symptoms of hypoxia) was highest on day 3 (2.13 ± 1.6) and it was below the cutoff criteria of 3.[15] A comparison of the scores between the baseline day and SD days showed an increasing trend; however, the same was not statistically significant. The scores provided an objective measurement of the severity of hypoxic exposure. However, the usage of the scores itself for quantifying short-duration exposures to hypoxia may not be a suitable option. Although symptoms of hypoxia may be seen on sudden exposure to high altitude, the LLAMS scores should be more appropriately used in a setting of longer exposure to altitudes since the questionnaire has been validated for assessing symptoms following 6 h of exposure to altitude.[15]
The means of time to 75% SpO2 (TTSPS), which was representing the hypoxia tolerance at that altitude[16] are given in [Table 2]. A comparison of time to 75% SpO2 across 4 days did not show any significant difference, thereby eliminating any influence of 2 h SD on the rate of desaturation of arterial blood. A study by Yoneda et al. used this parameter of arterial desaturation of oxygen to compare hypoxia tolerance in two age groups (those above and below 40 years of age) and found that the lower age group had longer times for fall in oxygen saturation.[17] In the present study, all participants were below the age of 40 years with a mean age of 33.53 ± 3.6 years which may explain the insignificant reduction in the time to 75% SpO2.
The HR and RR showed an increase in their means following acute exposure to altitude. This indicates the cardiovascular and respiratory response to the stress of hypoxia.[1] The difference in the means of HR on ground and altitude of 17,000 ft compared over 4 days showed no significant change. The effect of fatigue due to sleep loss did not show a quantifiable worsening in physiological parameters when exposed to hypoxic conditions.
The effect of SD on cerebral function is similar to that of hypoxia itself, with sleeplessness shown to decrease hypoxic and hypercapnic ventilatory responsiveness.[18,19] This is suggested by the decreased alpha activity,[7,8,18,20] the electroencephalographic wave of wakefulness seen during SD. Hypoxia tolerance is a known physiological balance between the cardiorespiratory compensation and the hypoxic insult. SD could be an influencing factor for hypoxia tolerance. However, 2 h of SD did not cause any significant effects on hypoxia tolerance. The duration of SD was probably not enough to bring such overwhelming effects of altered ventilatory responses. The absence of sleep could stress a person, thereby producing endogenous opiates, endorphins, in which animals have shown to decrease ventilatory responses.[21] However, endorphins have not shown to have such profound effects on adult human respiration, making this explanation unlikely. In a study by White et al., decrease in hypoxic ventilatory responses was seen with SD with measures of resting ventilation and arterial oxygen saturations remaining unchanged.[19] It is possible that the chemical control of breathing may be important in the maintenance of adequate ventilation and satisfactory arterial gas concentrations in these patients. The adverse effects of SD on these drives could have implications concerning the pathogenesis of acute-on-chronic episodes of hypoventilation and did not show any decrement in the oxygen saturations of healthy adult individuals exposed to short spells of reduced sleep in the present study.
A statistically significant increase in reaction times was noted for ST and DSST on exposure to altitude on all 4 days. However, a comparison of reaction times in all the tests did not show any significant difference across the 4 days indicating that the effect of “2 h SD” on cognitive performance under hypoxia condition could not be established. The effect of SD as a stressor sufficient enough to produce cognitive decrements was seen to be weaker than expected. A study by Dinges et al.[22] showed a significant cumulative increase in reaction time over 7 days when exposed to 5 h SD. A study by Elmenhorst et al.[10] showed a cumulative effect of SD on altitude exposure and similarities with cognitive impairments on blood alcohol concentration of 0.4–0.6%. Reduced duration of sleeping may still have some bearing on the performance abilities of an individual who is exposed to hypoxia since altitude has shown a definite decline whereas added SD was seen to be a weaker stressor. In safety-sensitive operational roles, the two stressors acting in conjunction may still show less than an optimum performance by aircrew. However, our study could not establish this fact, probably attributed to the absence of a sufficient level of cumulative sleep loss following 2 h of SD only for 2 days.
Although statistically not significant, an improvement in cognitive performance was seen when comparing the means of cognitive test responses on the day following recovery of sleep. This improvement could reflect that the sleep debt might have been partially recovered which needs further evaluation with larger sample size and longer duration of sleep loss.
Our study had few limitations. The Lake Louise Acute Mountain Sickness scores were used as a measure of the severity of symptoms of hypoxia exposure. The scale is ideally used in studies of altitude of longer duration. Furthermore, time to SpO2 of 75% was considered as hypoxia tolerance in this study. The reliability of finger pulse oximeters at oxygen saturations below 65–70% is questionable, and thus, the duration of exposure to hypoxia in subjects was restricted. A longer duration of hypoxia exposure may show more pronounced effects since the TUC at a chosen altitude of 17,000 ft is between 20 and 30 min.
CONCLUSION
The results of the study revealed that 2 h of SD on 2 consecutive nights did not alter the physiological parameters of HR, RR, and time to arterial desaturation to 75% SpO2 on exposure to 17,000 ft. Such exposure to 17,000 ft showed a significant slowing of responses in two cognitive tests (ST and DSST) on all days of study; however, the study could not elicit a statistically significant effect of 2 h of SD across 2 consecutive nights on the cognitive performance measures on exposure to hypoxia.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Hypoxia and hyperventilation In: Ernsting J, Rainford DJ, Gradwell DP, eds. Ernsting's Aviation and Space Medicine (5th ed). Florida, USA: CRC Press; 2016. p. :49-63.
- [CrossRef] [Google Scholar]
- Cognition at altitude: Impairment in executive and memory processes under hypoxic conditions. Aviat Space Environ Med. 2013;84:1159-65.
- [CrossRef] [PubMed] [Google Scholar]
- Sleep and performance. Am J Respir Crit Care Med. 2018;197:13-4.
- [CrossRef] [PubMed] [Google Scholar]
- AltitudeOmics: Decreased reaction time after high altitude cognitive testing is a sensitive metric of hypoxic impairment. Neuroreport. 2014;25:814-8.
- [CrossRef] [PubMed] [Google Scholar]
- Cognitive responses to hypobaric hypoxia: Implications for aviation training. Psychol Res Behav Manage. 2014;7:297-302.
- [CrossRef] [PubMed] [Google Scholar]
- Neuropsychological functioning associated with high-altitude exposure. Neuropsychol Rev. 2004;14:197-224.
- [CrossRef] [PubMed] [Google Scholar]
- The sleep-deprived human brain. Nat Rev Neurosci. 2017;18:404-18.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of sleep deprivation on cortical activation during directed attention in the absence and presence of visual stimuli. Neuroimage. 2011;58:595-604.
- [CrossRef] [PubMed] [Google Scholar]
- Lapsing when sleep deprived: Neural activation characteristics of resistant and vulnerable individuals. Neuroimage. 2010;51:835-43.
- [CrossRef] [PubMed] [Google Scholar]
- Performance impairment during four days partial sleep deprivation compared with the acute effects of alcohol and hypoxia. Sleep Med. 2009;10:189-97.
- [CrossRef] [PubMed] [Google Scholar]
- Sleep deprivation under sustained hypoxia protects against oxidative stress. Free Radic Biol Med. 2011;51:1842-8.
- [CrossRef] [PubMed] [Google Scholar]
- Fatigue in aviation: A survey of the awareness and attitudes of Indian air force pilots. Int J Aviat Psychol. 2007;17:275-84.
- [CrossRef] [Google Scholar]
- Sleep in America™ Poll. 2002. Washington, DC: National Sleep Foundation; Available from https://www.sleepfoundation.org/professionals/sleep-americar-polls/2002-adult-sleep-habits [Last accessed on 2021 Dec 23]
- [Google Scholar]
- Assessment of Fatigue among Air Force Aircrew by Employing Sleep Monitoring System, AFMRC Project No.4990/2018 Bangalore: IAM; 2018.
- [Google Scholar]
- The 2018 Lake Louise acute mountain sickness score. High Alt Med Biol. 2018;19:4-6.
- [CrossRef] [PubMed] [Google Scholar]
- Pulse oximetry at high altitude. High Alt Med Biol. 2011;12:109-19.
- [CrossRef] [PubMed] [Google Scholar]
- Time of useful consciousness in aircrew members with reference to prior altitude chamber experience and age. Aviat Space Environ Med. 2000;71:72-6.
- [Google Scholar]
- A review of the biological effects of total sleep deprivation in man. Biol Psychol. 1978;7:55-102.
- [CrossRef] [Google Scholar]
- Sleep deprivation and the control of ventilation. Am Rev Respir Dis. 1983;128:984-6.
- [Google Scholar]
- The EEG during prolonged experimental sleep deprivation. Electroencephalogr Clin Neurophysiol. 1962;14:544-51.
- [CrossRef] [Google Scholar]
- B-endorphin: Effects on respiratory regulation. Life Sci. 1978;23:1271-6.
- [CrossRef] [Google Scholar]
- Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4-5 hours per night. Sleep. 1997;20:267-77.
- [Google Scholar]






