Translate this page into:
Neurovestibular effects of +Gz
Abstract
The cardiovascular effects of +Gz are classically explained using the exaggerated hydrostatic column theory. This theory implied that blood supply to the brain is not compromised as long as ventricular driving pressure exceeds the hydrostatic column pressure. We studied the hypothesis that at relatively low +Gz levels, despite a mathematically predicted positive ventricular pressure gradient, slowing of blood flow may still reduce cerebral perfusion. Aberrant neurovestibular stimulation has long been known as a hallmark of vertebra-basilar insufficiency. We used Electro-Oculo-Graphy (EOG) as an easy indicator of neurovestibular responses and thus indirectly of relative cerebral ischemia, while the subjects (n=19) were subjected to +Gz in the human centrifuge for a period of 3 min. All 19 subjects developed a nystagmus during the build-up and decay phases of acceleration. This was a classical Lz nystagmus. In addition, 17 of the 19 subjects developed an intermittent, pathological nystagmus during steady state at +2.5 Gz, without any vestibular stimulation. This appears to be a consequence of ischemia and substantiates our hypothesis. The blood supply is compromised enough to cause functional decrement even at a relatively low +Gz value of + 2.5G.
Keywords
+Gz cerebral ischemia
Nystagmus
Human centrifuge
Cerebral and ocular ischemias have long been known as the most important effects of +Gz acceleration [1, 2]. The classic hydrostatic theory is used to explain these effects. This theory takes into account the hydrostatic pressure due to the column of blood above the level of the heart. The hydrostatic pressure is a product of height, density and +Gz of the column of blood and thus increases consequent to an increase in +Gz acceleration. This hydrostatic pressure opposes the pressure generated by the heart (mean arterial pressure). As the hydrostatic pressure increases, the height to which the heart can pump blood keeps decreasing, finally resulting in a loss of blood flow to the head and eyes [1,2]. Since the eyes have an internal pressure of approximately 20 mm Hg, the first symptoms are visual, i.e., a gray out or peripheral light loss (PLL) at about + 3.5 G [3]. It has been stressed that cerebral ischemia does not take place till after PLL develops [1, 2]. We would like to differ with this view.
The authors are of the opinion that before complete loss of blood supply, some slowing of circulation wouftl -take place. Such slowing would result in cerebral anemia, even though the mean arterial pressure is still higher than the opposing hydrostatic pressure. This study aims at investigating this hypothesis.
In this study we tried to answer two questions. Did cerebral anemia actually occur? If so, was it significant enough to cause a functional supply decrement? Neither of these was easy to answer. The decrement in blood supply was unlikely to be measurably low. Functional decrement was difficult to measure reliably. For these reasons, we approached the problem indirectly.
Vertebro-basilar insufficiency (VBI) has long been associated with a feeling of vertigo without any external vestibular stimulation [4, 5, and 6]. This indicates an ischemic malfunction of the vestibular system. Often this is the only symptom of VBI. This prompted us to believe that a study of the neurovestibular system may give us an idea of functionally significant decrement in the cerebral blood supply, even in the absence of other symptoms.
We studied the effects of relatively low +Gz acceleration on the neurovestibular system using binocular EOG, in healthy young human volunteers.
Material and Methods
The study was conducted after due approval by the Human Experimentation Committee of USAF School of Aerospace Medicine, Brooks AFB, Texas. Nineteen, healthy, male volunteers from the centrifuge panel at Human Systems Center at Brooks Air Force Base participated in this study. All volunteers were experienced centrifuge riders and had been subjected to +Gz on several occasions earlier. Care was taken to ensure that they had no current medical problem or recent illness. They had no vestibular symptoms. They were well rested on the day of the study. All volunteers had a light meal within 2-3 hours before the exposure. Their mean (+ SD) age was 28.5 Gt 4.44) yrs. All subjects wore anti-G suits, which were not inflated during the run.
The instrumentation consisted of routine, biaxial ECG electrodes that are mandatory for all human experimentation in the centrifuge at Brooks AFB. The EOG was recorded using bilateral, bipolar electrodes to measure both horizontal and vertical movements of both eyes, separately, over four channels. The electrodes were disposable, silver chloride, 0.5 cm in diameter. The electrode configuration is shown in Fig 1. The EOG data was collected using a digital EEG Monitor (Model A-1000, Aspect Medical Systems Inc., San Antonio, TX). The data was digitized and preamplifier inside the centrifuge gondola to minimize the effects of electrical noise on the signal. The data was collected at 128 samples per second.
Once the subject was instrumented and seated in the gondola, the signal was checked for quality using an impedance-measuring component of the system to ensure impedance lower than 10,000 ohms. For calibration, the subject was asked to look at the central red light and then, in turn, at the green lights on the light bar in the gondola those are at an angle of 25° to the subject. This was done repeatedly to ensure reproducibility. The subject was then asked to close his eyes to suppress fixation, and to keep his eyes closed throughout the experiment.
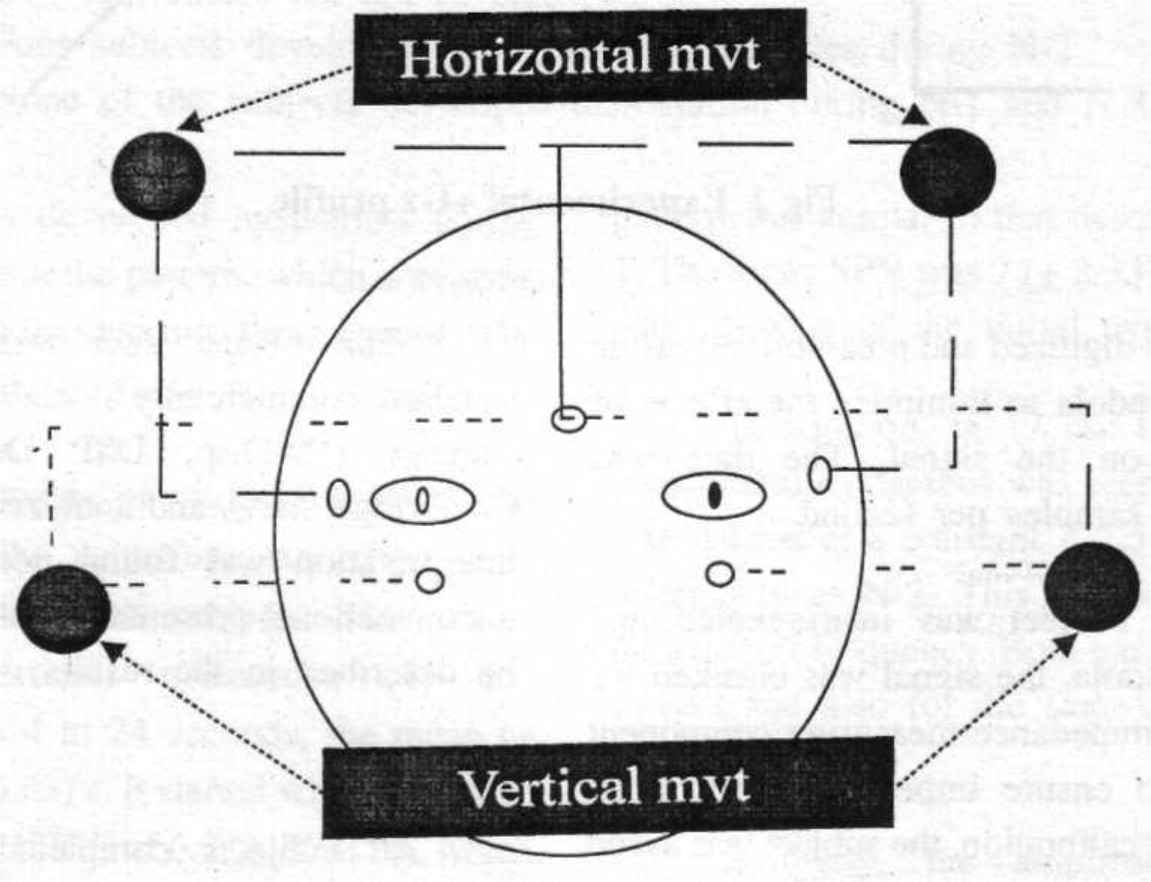
- Placement of electrodes
The centrifuge exposure consisted of a rapid (6 G/s) onset rate (ROR) to a peak value of + 2.5 Gz. The Subject stayed at peak + Gz level for 3 min (Fig 2). Eye movements were recorded throughout the centrifuge run.
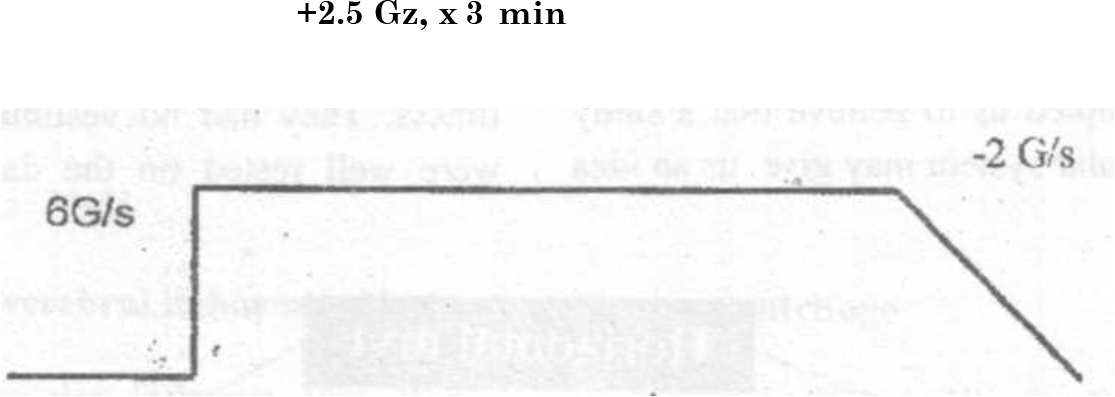
- Experimental +Gz profile
Following the experiment, the subject was required to remain seated with his eyes closed, and EOG recordings were continued till all eye movements returned to baseline.
The binary data was plotted using a standard, commercially available, medical graphics package (DADisp, DSP Development Corp., Cambridge, MA), and analyzed manually. Manual interpretation was found necessary due to the unconventional presentation of nystagmus, as will be described in the results.
Results
All subjects completed the experimental protocol successfully. One subject reported a slight nausea. All subjects reported a tumbling or turning sensation at the beginning and end of the run. No other symptoms were reported. All ECG changes were as expected. None of the subjects had symptoms of presyncope and there were no episodes of G-LOC.
All subjects developed nystagmus during the experiment. From the pattern, which was seen, the nystagmus was divided into three epochs. The results are tabulated in Table 1.
| Time of Nystagmus | Duration (mean + SD) | Slow Phase Velocity (mean + SD) | Present in N = 19 |
|---|---|---|---|
| During build up phase of acceleration (N-l) | 12.8 + 5.63 s | 6.8 ±1.74% | 19 |
| During Steady State at + 2.5 Gz (N-2) | Variable | Variable | 17 |
| During decay phase of acceleration (N-3) | 17.8 ± 8.36 s | 7 ±2.33% | 19 |
Note : Two individuals did not develop N-2
Four subjects developed dissociation of the eyes during N-2 None of the subjects developed dissociation during N-l and N-3
The first episode of nystagmus (N-l) was seen as soon as the centrifuge started moving. This was identified in all subjects. This was a classical Lz (vertical) nystagmus [8] and disappeared within 4 to 24 seconds, the mean (+ SD) being 12.8 (+ 5.63) s. It started with an average slow phase velocity (SPV) of 6.8 (+ 1.74) °/s. which gradually diminished over time until it subsided completely.
A similar nystagmus (N-3) was identified when the centrifuge started decelerating. This lasted for 4 to 34 seconds in different subjects, the mean (+ SD) duration being 17.8 (+ 8.36) s. This was also a classical Lz nystagmus. The frequency pattern was similar to that described above for N-1. The mean SPV was 7 (± 2.33) °/s. The direction was opposite of the initial nystagmus.
In addition, in 17 out of 19 subjects, an intermittent nystagmus was seen during the steady state period at a constant + 2.5 Gz. This shall be referred to as N-2. This did not have a uniform direction or frequency. Both varied from subject to subject and also for the same subject from time to time (Fig 3).
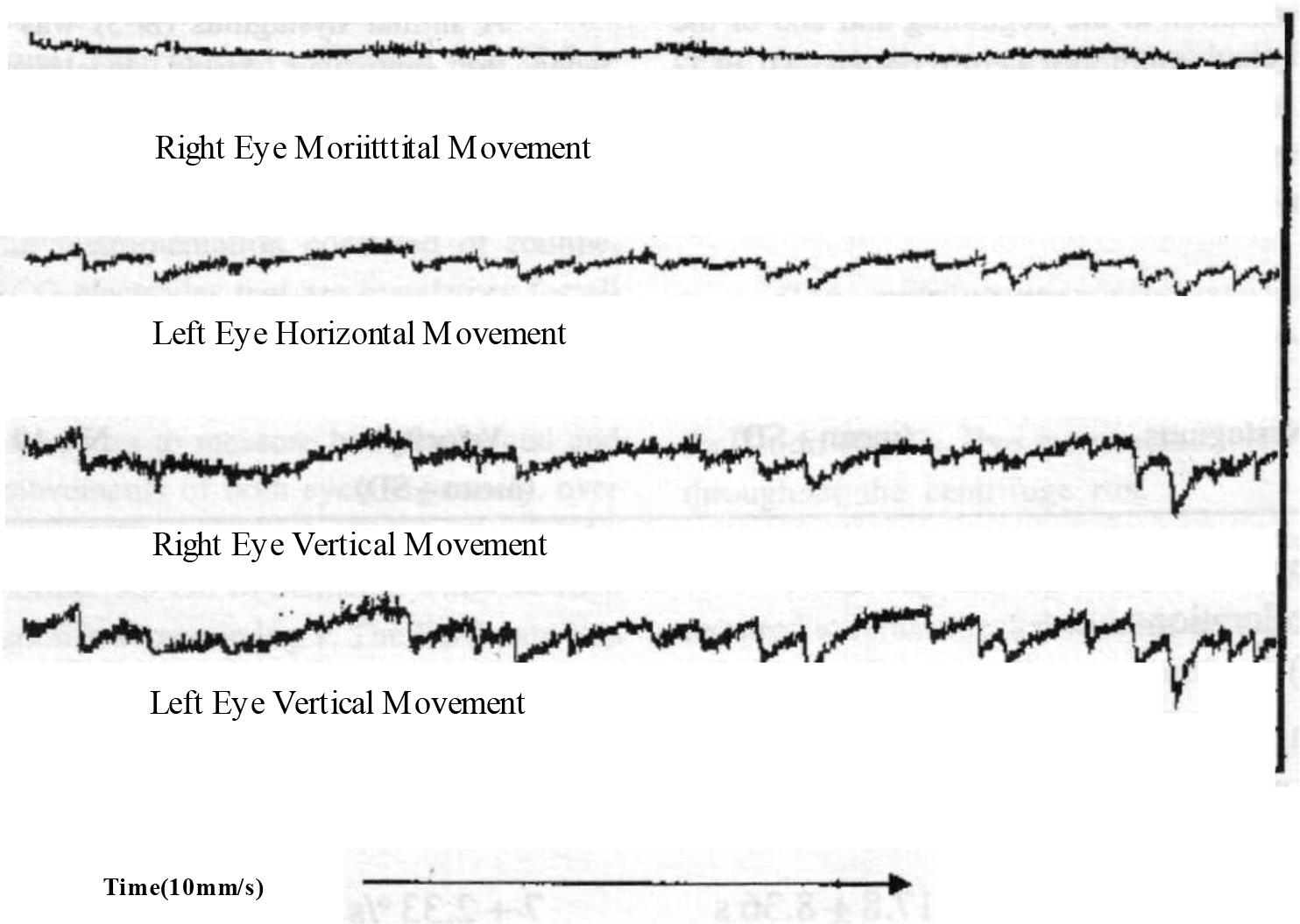
- Tracing showing intermittent nystagmus
- Note : Tracing shows both eyes moving simultaneously. The movement is predominantly vertical with a slight horizontal component. The amplitude, frequency and slow phase velocity of the nystagmus changes with time.
Similarly, the amplitude of the slow component and consequently the velocity kept varying from time to time. The slow phase velocity varied from 0.7 to 2 °/s. In four subjects the two eyes were seen to be dissociated and thus moving separately (Fig 4).
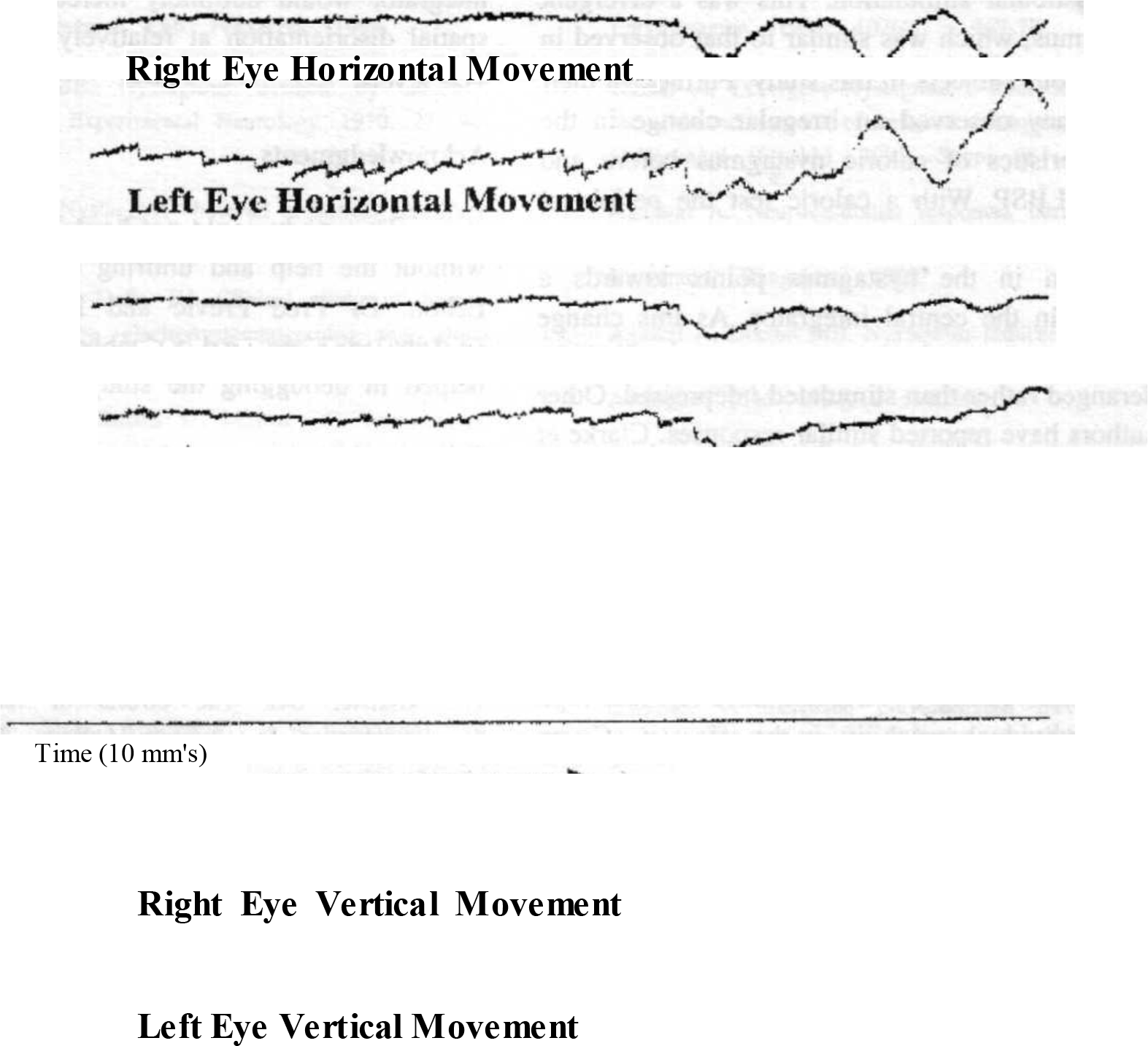
- Tracing showing dissociation
- Note : Tracing showing dissociation of the two eyes. The left eye is showing a predominantly horizontal nystagmus, while the right eye remains stationary. It is noteworthy that both eyes show a slow drifting movement together, indicating that the system is functioning properly.
Discussion
The normal ECG responses indicate that the subjects reacted normally to the + Gz stress.
The nystagmus at the beginning and end of the run is as has been described by others [7, 8]. This was associated with a tumbling or turning sensation as is expected. The frequency pattern and duration of this nystagmus is the same as that elicited by Gerandt [9] using direct utricular stimulation.
The nystagmus during the steady state period (N-2) was different in character. It had no definite direction, frequency or slow phase velocity. It had no identifiable pattern. Cheung has observed a similar nystagmus [10], in his studies at DCIEM, using infrared video photography.
We feel that the key to understanding lies in determining the cause and origin of this nystagmus. McGarth et al have described a vertical nystagmus during a steady state at + 3 Gz [8], in their studies on the human centrifuge. They attributed this to the constant stimulation of the utricular macula due to high + Gz levels. This reasoning is not borne out by studies done by Gernandt [9], which clearly demonstrated that a nystagmus induced by constant utricular stimulation subsides over time.
Also, nystagmus developing from the peripheral vestibular organs has definite characteristics. It is similar in both eyes, has a definite reproducible frequency and waveform characterized by the slow wave velocity [11, 12, 13]. It usually follows a stimulation / pathology of the peripheral vestibular organ.
On the other hand the nystagmus seen in our study is irregular, is associated with a changing frequency and waveform, and is intermittently brought about without any demonstrable stimulus and at times, results in disconjugate movement of the eyes. This type of a presentation is associated with a pathological central nystagmus [6, 11, 12, 13]. Thus we feel inclined to believe this nystagmus to be of central (brain stem, cerebellum) rather than peripheral (otolith, semi circular canal) origin.
A direction changing nystagmus has been associated with vertebrobasilar insufficiency [4, 6]. Divergence is very rare in nature, with only two cases reported in clinical literature so far [14, 15]. These have also been attributed to brainstem vascular pathology [14, 15]. It is thus felt that the four cases, which were seen in our study, are important and cannot be disregarded.
Further proof that the nystagmus was a result of a diminished cerebral blood flow and not due to peripheral vestibular stimulation come from the work of Agarwal and Dikshit [16, 17] who recorded a similar bizarre nystagmus during cerebral ischemia induced by lower body subatmospheric pressure (LBSP) in the absence of any vestibular stimulation. This was a divergent nystagmus, which was similar to that observed in four of our subjects in this study. Further, in their study they observed an irregular change in the characteristics of caloric nystagmus before and during LBSP. With a caloric test the peripheral vestibular stimulation is controlled and hence the alteration in the nystagmus points towards a change in the central integrator. As this change was bizarre, the central integrator appears to be deranged rather than stimulated / depressed. Other authors have reported similar responses. Clarke et al [18] have reported a change in the caloric nystagmus during + 3 Gz, and have reported high inter-individual variability in the effect of +Gz on the caloric nystagmus as reported by Agarwal and Dikshit. It may be mentioned here that most authors mentioned above did not perform binocular nystagmography, and therefore could not comment upon divergence.
The above discussion points towards a breakdown of the central vestibular integrator under +Gz. Since a similar change has been seen with LBSP, and the only common thing between the two stressors is a decreased blood supply, the authors are of the belief that central cerebral ischemia is the primary cause of this breakdown.
It is noteworthy that this breakdown took place without any subjective vestibular symptoms. It occurred at levels of + 2.5 Gz where a decrement of cerebral blood supply is not expected and in the absence of visual symptoms, which are expected before cerebral circulation is compromised. This decrement to blood supply is potentially dangerous. While it remains symptomless, there is a definite change in cerebral functioning, which may result in errors of judgment. Also spatial disorientation is common in scenarios where prolonged turning and hence +Gz is involved. An altered perception by the central vestibular integrator would definitely increase the risk of spatial disorientation at relatively low sustained +Gz levels.
Acknowledgments
This study could not have been completed without the help and untiring efforts of Roger Echon. Dr Fred Previc and Dr Ken Stevens contributed ideas and valuable critique, which helped in debugging the study.
References
- The effects of long duration acceleration In: Aviation Medicine In: Ed Emsting J King P (2nd edition). 1988. p. :139-59.
- [Google Scholar]
- Biodynamic: Sustained acceleration In: Fundamentals of Aerospace Medicine (2nd edition). Baltimore: Ed DeHart RL William and Wilkins; 1996. p. :201-60.
- [Google Scholar]
- Reproducibility of +Gz tolerance testing. Aviat Space Environ Med. 1979;50(8):825-8.
- [Google Scholar]
- Apo geotropic type of direction changing nystagmus related to slow vertebra-basilar blood flow. Acta Otolaryngol (Stock). 1995;520(Supple):350-3.
- [Google Scholar]
- Therapeutically clinical models using the lower body negative pressure chamber for simulating vertebro-basilar insufficiency syndromes in humans. Acta Otolaryngol (Stock) 1991(Suppl 481):548-50.
- [Google Scholar]
- The top diagnostic value of direction of cervical nystagmus in vertebro-basilar insufficiency. (English Abstract) Otolaryngol Pol. 1994;48(5):478-82.
- [Google Scholar]
- Vestibulo-ocular responses in man to +Gz hyper gravity. Aviat Space Environ Med. 1990;61:631-5.
- [Google Scholar]
- Human vestibulo-ocular response during +3Gz centrifuge stimulation. Report NAMRL-1388 Naval Aerospace Medical Research Lab. Pensacola.
- [Google Scholar]
- Clinical study of human equilibrium by electronystagmography and allied tests. Popular Prakashan Bombay.
- [Google Scholar]
- Divergent Nystagmus: Recordings from an advanced system of infrared oculography. Acta Otolaryngol (Stockh) 1991(Suppl 481):451-9.
- [Google Scholar]
- Neurovestibular responses during lower body sub atmospheric pressure. Bangalore: MD thesis Bangalore University;
- [Google Scholar]
- Nystagmus induced by lower body suction. Proceedings of 42"* ICASM. Ind J Aerospace Med. 1994;2:52-4.
- [Google Scholar]
- Three dimensional aspects of caloric nystagmus in humans: I. The influence of increased gravitoinertial force. Acta Otolaryngol (Stockh). 1993;113:687-92.
- [Google Scholar]






