Translate this page into:
Effect of Zolpidem on sleep efficiency and heart rate during daytime nap

*Corresponding author: Dr. (Prof.) B Sinha, Scientist ‘E’, Department of Space and Environment Physiology, Institute of Aerospace Medicine IAF, HAL Old Airport Road, Vimanapura, Bengaluru - 560 017, Karnataka, India. bsinha2001@yahoo.co.in
-
Received: ,
Accepted: ,
How to cite this article: Sinha B, Yadav CT. Effect of Zolpidem on sleep efficiency and heart rate during daytime nap. Indian J Aerosp Med 2019;63(2):83-9.
Abstract
Introduction:
Zolpidem is a non-benzodiazepine and a type-A gamma-aminobutyric acid (GABA) receptor agonist of the imidazopyridine class of drugs which acts as a short-acting sedative and hypnotic. It induces sleep by augmenting its effect in the central nervous system, by binding to GABAA receptor. The drug has been approved for use in USAF and others to induce sleep after extended duration combat mission. Studies are rare where the impact of drug on daytime nap has been studied. In extended duration operation, necessity of inducing sleep to the combatant after the mission is extremely important and unequivocal. The present study was undertaken to observe the impact of Zolpidem on sleep efficiency (SE) and autonomic heart rate (HR) response during short-term daytime nap.
Material and Methods:
In a double-blind repeated measure, randomly ordered design, 20 healthy male volunteers were evaluated for SE and HR during daytime nap after administering two different doses of Zolpidem. The study protocol involved the analysis of SE using polysomnographic recordings. The participants were advised to report to their normal place of work and do only routine works not involving strenuous activities. They were advised to report to the sleep laboratory at 1200 h. Three different sessions were carried out on each participant, keeping a gap of 72 h between two sessions. The participants were allowed to sleep in the sleep laboratory in a thermoneutral and sound-attenuated condition. They were permitted to sleep ad libitum. The participants were tested without drug in one of the sessions and during other two sessions, the participants were given 5 mg or 10 mg Zolpidem. One-way ANOVA was carried out to analyze the data and Tukey honestly significant difference post hoc test was employed to compare between the three conditions.
Results:
Total sleep time (TST) increased significantly from 120.1 ± 38.34 min at baseline to 232.3 ± 41.30 min (P < 0.001) after 5 mg Zolpidem and 249.2 ± 26.92 min after 10 mg Zolpidem (P < 0.001). SE (calculated by dividing the amount of time spent asleep [in min] by the total amount of time in bed [in min]) increased significantly from baseline to post-Zolpidem administration (79 ± 11.52%, 91.5 ± 3.61%, and 94.5 ± 1.59%) (P < 0.001 as compared from baseline data). Average HR (AHR) decreased during sleep from baseline (88.0 ± 8.68 bpm) to 5 mg Zolpidem administration (78.2 ± 7.42) (P < 0.001) and to 10 mg Zolpidem administration (79.2 ± 7.33 bpm) (P < 0.01).
Conclusion:
Zolpidem administration caused an increase in TST and SE and a decrease in AHR during daytime nap.
Keywords
Zolpidem
Total sleep time
Sleep efficiency
Heart rate
INTRODUCTION
In modern combat scenario, aircrew and other military personnel may have to work for extended durations. The detrimental effects of fatigue that emerges from lack of sleep and long working hours may adversely affect the physical and mental performance of the personnel. Fatigued individuals suffer from increased lethargy, reduced cooperativity, and impaired cognitive functions which ultimately may make the future mission unsafe and compromised.[1] The importance of restful sleep following a long-duration mission is unequivocal and helps to restore physiological reserve of the personnel. Considering the mission criticality, it is an undeniable fact that initiation of sleep with rapidity is of utmost importance for better rescheduling of work-rest regime for the troops/ aircrew.
Several pharmacological interventions have been tried to induce rapid onset of sleep in combat operations. Zolpidem is one such drug which is a short-acting hypnotic and sedative. It is a non-benzodiazepine and falls under imidazopyridine class of drugs.[2] It induces sleep by augmenting its effect in the central nervous system (CNS), by binding with gamma-aminobutyric acid (GABAA) receptor. The drug is preferred over benzodiazepine class of drugs as it is rapid in onset, has a short half-life, can preserve sleep architecture, and has a lower risk of tolerance.[3] The drug has been approved for use in USAF and others to induce sleep after extended duration combat mission. Zolpidem is a “No-Go” pill which has a beneficial effect in initiation and maintenance of sleep without subsequently affecting alertness and performance. It has been approved for limited use in military aircrew in India during sustained operations.
Zolpidem showed a promising effect on sleep quality and fatigue management during surge operations in Pilots of Remotely Piloted Aircraft.[4] Considering Zolpidem short half-life of 1.5–3.2 h, it may be effectively utilized for short-term management of sleep problems.[3] The effect of Zolpidem on cardiovascular parameters during nighttime sleep was studied in healthy middle-aged males where it was found that Zolpidem had no effect on heart rate (HR) in comparison to placebo.[5] Studies are rare where the effect of Zolpidem on autonomic cardiovascular variables during daytime sleep has been examined. The present study aimed to examine the impact of two different doses of Zolpidem administration on autonomic HR response, total sleep time (TST), and sleep efficiency (SE) in healthy Indian males during daytime nap in a double-blind, randomized and repeated measure study.
MATERIAL AND METHODS
A double-blind, randomized, repeated measure design was employed to evaluate the effect of Zolpidem in 20 healthy male volunteers. Inclusion criteria included healthy males in the age range of 20–40 years. Exclusion criteria for the selection of volunteers were a history of any sleep disturbance or psychiatric disorders, inadequate sleep in the previous night, history of any drug or medicine intake, smokers or any disease or infirmity. They were explained in detail the protocol of the study and possible side effects of intake of Zolpidem. A written informed consent was obtained from each participant and the Ethical Committee of the Institute approved the test protocol.
The study protocol involved recording of sleep and HR data during daytime sleep on three different occasions with a gap of 72 h between two sessions. Baseline polysomnographic and electrocardiographic data were recorded in one session. The other two sessions involved recording of physiological data after administering either 5 mg or 10 mg of Zolpidem. The order of the experimentation was in random order. The Zolpidem was taken per oral by the individuals and went to the bed immediately. The participants were allowed to sleep ad libitum. The time spent in the bed and TST were measured from the polysomnographic recordings. TST was measured from polysomnographic data such as electroencephalography (EEG), electrooculography, and limb movement data. SE was calculated by dividing the amount of time spent asleep (in min) by the total amount of time in bed (in minute) during three experimental procedures.
Three electrodes were placed on the anterior surface of the chest for recording single-lead electrocardiogram (ECG) to obtain HR data. HR data were recorded during the entire sleep duration. Average HR (AHR), highest HR (HHR), and lowest HR (LHR) during sleep were measured from electrocardiographic data.
Baseline EEG of the subjects was recorded prior to the day of the study to assess the normal sleep pattern. On the day of study, the participants were advised to report to their normal place of work and do only routine works not involving any strenuous activities. They reported to the sleep laboratory in the Department of Physiology at 1200 h. The study was conducted in a controlled temperature and sound attenuated environment of the sleep laboratory. Throughout the study, the participants and the observers were blinded. The data were first checked for normality by Shapiro–Wilk “W” statistical tool and were observed to be normally distributed. Hence, parametric statistical analysis one-way ANOVA was carried out for the analysis of interval data. Non-parametric tests such as Cochran’s Q test and McNemar’s test were carried out to examine whether the proportion of the HR in individuals varied between baseline and Zolpidem administration. The level of significance was kept at P < 0.05.
RESULTS
The study group comprised 20 healthy male volunteers with a mean age, height, and weight of 31.1 ± 5.69 years, 170.5 ± 4.56 cm, and 69.8 ± 3.25 kg, respectively.
Table 1 shows TST and SE data at baseline and after administration of 5 mg and 10 mg Zolpidem.
| Baseline | 5 mg Zolpidem | 10 mg Zolpidem | |
|---|---|---|---|
| TST (in min) | 120.1±38.34 | 232.3±41.29*** | 249.2±26.92*** |
| SE (in %) | 79.0±11.52 | 91.5±3.60*** | 94.5±1.59***,$$$ |
TST was found to be significantly higher after 5 mg (P < 0.001) and 10 mg (P < 0.001) of Zolpidem administration as compared to baseline.
Figure 1 shows the AHR, LHR, and HHR during daytime sleep without Zolpidem and with two different doses of Zolpidem. There was a generalized decrease in HR during sleep after intake of Zolpidem. The AHR decreased significantly from baseline (88.0 ± 8.68 bpm) to post-Zolpidem intake (78.2 ± 7.42 bpm after 5 mg and 79.21 ± 7.32 bpm after 10 mg, both being significant at P < 0.001). The LHR also decreased significantly after Zolpidem administration (60.9 ± 9.19 bpm at baseline to 55.1 ± 9.35 bpm post 5 mg [P < 0.001] and 50.4 ± 9.65 bpm post 10 mg [P < 0.001]). The HHR decreased significantly during sleep after 5 mg of Zolpidem administration as compared to control condition (114.4 ± 10.44 bpm to 102.6 ± 6.87 bpm, P < 0.001). The HHR decreased during sleep significantly after 5 mg of Zolpidem (114.4 ± 10.44 bpm to 102.6 ± 6.87 bpm). However, the HHR fall after 10 mg of Zolpidem administration (110.1 ± 7.57 bpm) was not statistically significant as compared to control. The AHR during sleep without the drug was found to be more than 80 beats/min in 95% of the individuals, but AHR reduced in number of individuals significantly after Zolpidem administration (40% and 50% had >80 bpm after 5 and 10 mg Zolpidem administration). For estimating the proportion of individuals who had AHR more than 80 beats/min, a Cochran’s Q test was run. Cochran’s Q test was carried out to determine whether Zolpidem had a significant impact on AHR. The test was applied on the dichotomous dependent nominal variable during three conditions baseline, 5 mg and 10 mg of Zolpidem administration keeping a cutoff of HR at 80 beats/min. Table 2 shows the output of Cochran’s Q test.
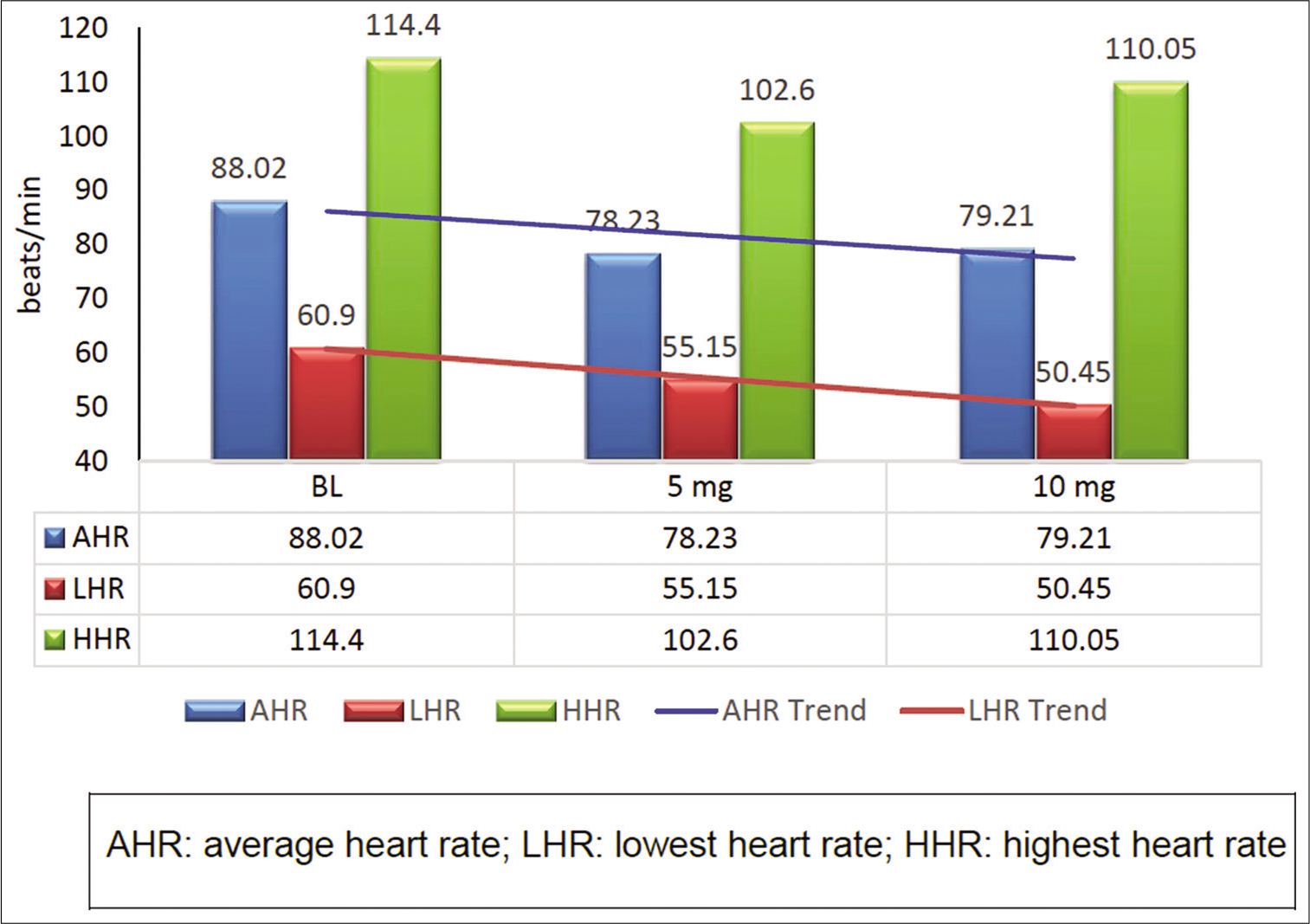
- The effect of Zolpidem on heart rate during daytime nap.
| Condition | Heart rate > 80 bpm | Heart rate < 80 bpm | Cochran’s Q and level of significance |
|---|---|---|---|
| Baseline | 19 | 1 | 14.714; P=0.001 |
| 5 mg | 8 | 12 | |
| 10 mg | 10 | 10 |
Following a significant outcome from Cochran’s Q test, McNemar’s test was carried out for post hoc analysis for individual comparisons. Non-parametric test like McNemar’s test was applied (for paired nominal data having binomial distribution) to find the significant change in proportion of AHR between baseline and 5 mg Zolpidem administration, between baseline and 10 mg Zolpidem and 5 mg and 10 mg Zolpidem administration. A 2 × 2 contingency table was created in all 3 times (keeping an HR cutoff at 80 beats/min). Table 3 shows the McNemar’s test of significance for AHR between baseline and 5 mg Zolpidem administration. The result showed that there existed a statistically significant change in proportions of AHR between baseline and 5 mg of Zolpidem administration (P < 0.039).
| AHR at baseline | AHR at 5 mg | McNemar’s test of significance | |
|---|---|---|---|
| HR>80 bpm | HR<80 bpm | ||
| HR >80 bpm | 8 | 11 | 0.001 |
| HR <80 bpm | 0 | 1 | |
AHR: Average heart rate, HR: Heart rate
The outcome of McNemar’s post hoc test between baseline and 10 mg Zolpidem is shown in Table 4. There was a statistically significant difference in proportion of the heart AHR between baseline and 10 mg Zolpidem administration (P < 0.004).
| AHR at baseline | AHR at 10 mg | McNemar’s test of significance | |
|---|---|---|---|
| HR>80 bpm | HR<80 bpm | ||
| HR >80 bpm | 10 | 9 | 0.004 |
| HR <80 bpm | 0 | 1 | |
AHR: Average heart rate, HR: Heart rate
Table 5 shows the significance level between 5 and 10 mg of Zolpidem administration.
| AHR at baseline | AHR at 10 mg | McNemar’s test of significance | |
|---|---|---|---|
| HR>80 bpm | HR<80 bpm | ||
| HR >80 bpm | 5 | 3 | 0.727 |
| HR <80 bpm | 5 | 7 | |
AHR: Average heart rate, HR: Heart rate
The LHR data were analyzed using non-parametric test Cochran’s Q test. The outcome of the test result is shown in Table 6. The impact of Zolpidem on LHR was also evident as the result was significant (P = 0.000).
| Condition | Heart rate >55 bpm | Heart rate <55 bpm | Cochran’s Q and level of significance |
|---|---|---|---|
| Baseline | 19 | 1 | 22.533; P=0.000 |
| 5 mg | 11 | 9 | |
| 10 mg | 4 | 16 |
Post hoc McNemar’s test was carried out after a significant outcome in Cochran’s Q test for individual comparisons. Tables 7-9 show the comparison between baseline and 5 mg, baseline and 10 mg, and 5 mg and 10 mg, respectively. There existed a significant difference between baseline and Zolpidem administration (refer tables for significance level).
| LHR at baseline | LHR at 5 mg | McNemar’s test of significance | |
|---|---|---|---|
| HR>55 bpm | HR<55 bpm | ||
| HR >55 bpm | 11 | 8 | 0.008 |
| HR <55 bpm | 0 | 1 | |
LHR: Lowest heart rate, HR: Heart rate
| LHR at baseline | LHR at 10 mg | McNemar’s test of significance | |
|---|---|---|---|
| HR>55 bpm | HR<55 bpm | ||
| HR >55 bpm | 4 | 15 | 0.008 |
| HR <55 bpm | 0 | 1 | |
LHR: Lowest heart rate, HR: Heart rate
| LHR at 5 mg | LHR at 10 mg | McNemar’s test of significance | |
|---|---|---|---|
| HR>55 bpm | HR<55 bpm | ||
| HR >55 bpm | 4 | 7 | 0.016 |
| HR <55 bpm | 0 | 9 | |
LHR: Lowest heart rate, HR: Heart rate
The HHR data also showed a significant difference in proportion of the HR that showed a significant fall (Cochran’s Q: 8.375; P < 0.015). However, the post hoc test (McNemar’s test) revealed the significant difference only between baseline and 5 mg Zolpidem administration (P = 0.004).
DISCUSSION
Zolpidem is a non-benzodiazepine short-acting sedative and hypnotic. It is a Type A GABA receptor agonist of the imidazopyridine class.[6] The drug is rapidly absorbed from gastrointestinal system with a Tmax of about 1 h and has a plasma half-life of 2.5 h or less.[7] This GABA receptor agonist decreases the time to sleep onset by about 15 min by increasing the GABA concentration in the CNS.[6,8] Zolpidem has been approved for use in USAF as a fatigue countermeasure.[9] Republic of Singapore Air Force has approved the use of Zolpidem for sleep management in military aircrew since 2005.[10]
The results of the present study indicated the significant impact of Zolpidem administration on SE and modulation of autonomic cardiovascular parameter, namely, HR during short duration day nap. There was an increase in SE on Zolpidem administration. Furthermore, there was a marked reduction in HR during sleep after Zolpidem administration.
Zolpidem is the most commonly prescribed sleep-promoting agent. The study has indicated that Zolpidem is effective in promoting sleep onset and increases TST at doses of 10 mg or higher both in subjective and laboratory-based sleep studies.[11] The increase in SE and TST after administration of Zolpidem, as observed in the present study, may be attributed to Zolpidem ability to reduce sleep latency.[12] However, the additional beneficial effect of higher dosage of Zolpidem on sleep parameters was not demonstrated in the present study. TST was increased over the baseline value by 93% after 5 mg Zolpidem intake and 107% after 10 mg of Zolpidem intake. In absolute term, the sleep time differed by a mere 17 min between low and high doses of Zolpidem. SE also did not increase by a higher proportion on application of higher dose of Zolpidem. It was increased only by 3% after a higher dose of Zolpidem as compared to low dose (91% vs. 94%).
The HR decreases by 5–8% during non-rapid eye movement (NREM) sleep and shows a wide fluctuation during REM sleep. Bradycardia during NREM sleep results from a tonic increase in parasympathetic activity. In another experimental study, it was observed that sympathectomy had little effect in HR reduction during NREM sleep.[13-16]
However, during REM sleep, bradycardia becomes more intense due to tonically reduced sympathetic discharge. Overall, parasympathetic activity predominates during sleep. The slowing down of HR during sleep due to enhanced parasympathetic neural drive was also confirmed by spectral analysis of HR variability indices. The predominance of high-frequency wave (0.15–0.4 Hz) in the ECG during sleep confirms this fact. The occurrence of bradycardia during sleep was also corroborated by examining baroreflex sensitivity (BRS) in literature.[17,18] BRS is a measure of autonomic cardiovascular function and is defined as the time of RR interval in the ECG (in millisecond) per unit change in systolic/diastolic blood pressure measured either during hypertensive-bradycardia sequence or hypotensivetachycardia sequence.[19] Increase in BRS is an indication of parasympathetic dominance. Several studies have observed an increment of BRS during sleep, particularly in NREM sleep.[17,18]
The nucleus tractus solitarius (NTS), a central station in the autonomic neural network, is located in dorsal region of the medulla and is responsible for autonomic cardiovascular regulation. The final pathway of sympathetic neural network within the medulla is mediated from NTS through caudal ventrolateral medulla (CVLM) and intermediate ventrolateral medulla (IVLM) to rostral ventrolateral medulla (RVLM). The neurotransmitter involved in the pathway between CVLM and IVLM to RVLM is GABA. In addition, the medullary vagal nuclei, namely, dorsal motor nucleus of vagus and nucleus ambiguus also receive inputs from higher cortical centers such as hypothalamus and amygdala [Figure 2]. The descending projections to vagal nuclei also modulate the vagal mediated slowing down of HR during sleep. GABA is the most potent inhibitory neurotransmitter found in the brain. Three subtypes of GABA receptors have been identified. These are GABAA, GABAB, and GABAC. GABAA receptor is a pentameric subunit complex which surrounds a central chloride channel. The GABAA and GABAB are mostly found in the CNS, whereas GABAC is exclusively found in the retina. GABAA receptors are widely distributed throughout the human brain and spinal cord and are activated by GABAergic inhibitory system that exists throughout the human CNS. The inhibitory effect of GABA receptor is thought to be achieved through increased influx of negative ion like Cl−, decreased influx of positive ion like Ca++ along with an increased efflux of K+.[20] Zolpidem exerts its action by binding with α1 subunit of GABAA receptor in the CNS. On activation, GABAA receptor allows chloride ion to flow into the cells which results in neuronal hyperpolarization.[21] The ventrolateral preoptic nucleus (VLPO) of anterior hypothalamus contains neurons that secrete GABA and galanin. The reticular activating system (RAS) spread through pons to midbrain contains two distinct sets of neuronal networks that are projected to the cerebral cortex directly or indirectly through thalamus [Figure 2]. RAS is made up of neural clusters that contain serotonergic, noradrenergic, adrenergic, and cholinergic nerve fibers. Some of the prominent nuclei in the RAS system include dorsal raphe nucleus, the locus coeruleus, and the pontine cholinergic system. GABA system interacts extensively with pontine noradrenergic and cholinergic system in modulating the sleep-wake cycle. Experimentally, it has been observed that local administration of GABAA receptor agonist increases REM sleep. On the other hand, local microinjection of serotonin receptor agonists induces wakefulness.[22] The interaction between the VLPO and the branches of the ascending RAS is mutually inhibiting to each other.[23]
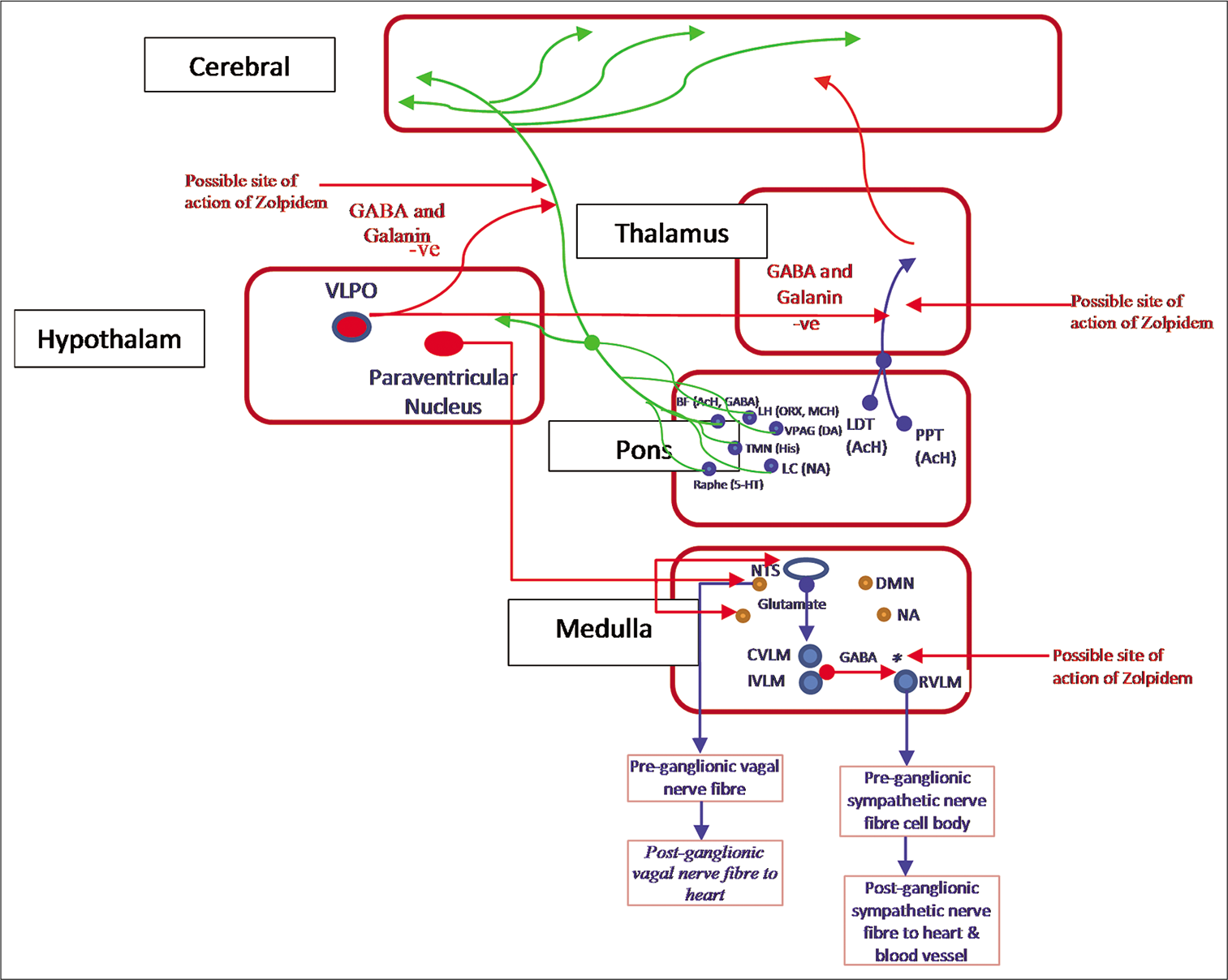
- Reticular activating system comprising two distinct sets of neural networks.
First cholinergic branch projects to the thalamus where they enhance the arousal of the cerebral cortex to sensory information relayed from thalamus. A second pathway activates the cerebral cortex to facilitate the processing of inputs from the thalamus. PPT: Pedunculopontine nucleus; LDT: Laterodorsal tegmental nucleus; BF: Basal forebrain; LC: Locus coeruleus; NA: Noradrenaline; TMN: Tuberomammillary nucleus; His: Histamine; LH: Lateral hypothalamus; ORX: Orexin; MCH: Melanin-concentrating hormone; BF: Basal forebrain; VLPO: Ventrolateral preoptic nucleus of hypothalamus. Also shown in the figure, medullary cardiovascular center comprising vagal nuclei-like DMN: Dorsal motor nucleus, NA: Nucleus ambiguus; and sympathetic nuclei-like CVLM: Caudal ventrolateral medulla; IVLM: Intermediate ventrolateral medulla; RVLM: Rostral ventrolateral medulla, a nucleus in hypothalamus.
It may be conjectured that Zolpidem after binding with GABAA receptor in VLPO region may augment the inhibitory action of GABA. Furthermore, Zolpidem after binding with GABAA receptor at postsynaptic membrane of RVLM possibly inhibits the neural outflow in the sympathetic system, thus causing intense bradycardia during sleep. The average LHR during sleep fell to 50 bpm in individuals after 10 mg of Zolpidem intake in the present study. In addition, the other excitatory inputs from higher centers of brain to NTS during sleep can also cause an inhibition to RVLM which results in reduced sympathetic drive.
CONCLUSION
Zolpidem in its low dose is efficacious in improving SE and sleep time. This has got tremendous importance in extended duration ops scenario where aircrew needs to have a restful and quality sleep for few hours to get ready for the successive missions. In addition, the higher dose of Zolpidem did not render any additional benefit as far as SE and sleep time are concerned.
Acknowledgment
The author is thankful to the residents in Aerospace Medicine who volunteered to participate in the study. Thanks are due to Medical Assistants of the department who assisted in recording of EEG during sleep and make the laboratory ready for the experimentation.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Physiology of sleep and wakefulness, sleep disorders and the effects on aircrew In: Gradwell DP, Rainford DJ, eds. Ernsting's Aviation and Space Medicine (5th ed). Florida, USA: Taylor and Francis Group, CRC Press; 2016. p. :245-61.
- [Google Scholar]
- Sleep deprivation and excessive daytime sleepiness. Sleep Disorders Medicine 2017:29-39.
- [CrossRef] [Google Scholar]
- Effect of Zolpidem on sleep quality of professional firefighters; a double blind, randomized, placebo-controlled crossover clinical trial. Acta Med Iran. 2015;53:573-8.
- [Google Scholar]
- Zolpidem in fatigue management for surge operations of remotely piloted aircraft. Aviat Space Environ Med. 2009;80:553-5.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of Zolpidem during sleep on ventilation and cardiovascular variables in normal subjects. Fundam Clin Pharmacol. 1993;7:305-10.
- [CrossRef] [PubMed] [Google Scholar]
- Development of subtype-selective GABAA receptor compounds for the treatment of anxiety, sleep disorders and epilepsy. GABA and Sleep 2010:25-72.
- [CrossRef] [Google Scholar]
- Zolpidem as a sleep aid for military aviators. Aerosp Med Hum Perform. 2018;89:406-8.
- [CrossRef] [PubMed] [Google Scholar]
- A multicenter, placebo-controlled study evaluating Zolpidem in the treatment of chronic insomnia. J Clin Psychiatry. 1994;55:192-9.
- [Google Scholar]
- Treating insomnia: Current and investigational pharmacological approaches. Pharmacol Ther. 2006;112:612-29.
- [CrossRef] [PubMed] [Google Scholar]
- Alterations of autonomic functions during sleep In: Lowey AD, Spyer KM, eds. Central Regulation of Autonomic Functions. New York: Oxford University Press; 1990. p. :367-92.
- [Google Scholar]
- Human heart rate variability and sleep stages. Ital J Neurol Sci. 1996;17:437-9.
- [CrossRef] [PubMed] [Google Scholar]
- Heart rate variability in normal and pathological sleep. Front Physiol. 2003;16:294.
- [Google Scholar]
- Baroreflex modulation during sleep and in obstructive sleep apnea syndrome. Auton Neurosci. 2012;169:7-11.
- [CrossRef] [PubMed] [Google Scholar]
- Cardiovascular autonomic dysfunctions and sleep disorders. Sleep Med Rev. 2016;26:43-56.
- [CrossRef] [PubMed] [Google Scholar]
- Baroreflex sensitivity: Measurement and clinical implications. Ann Noninvasive Electrocardiol. 2008;13:191-207.
- [CrossRef] [PubMed] [Google Scholar]
- Neurotransmitters and neuromodulators In: Ganong's Review of Medical Physiology (26th ed). USA: McGraw-Hill Education; 2019. p. :333-76.
- [Google Scholar]
- New insights into the role of the GABA(A)-benzodiazepine receptor in psychiatric disorder. Br J Psychiatry. 2001;179:390-6.
- [CrossRef] [PubMed] [Google Scholar]
- Interactions between GABAergic and serotonergic processes in the dorsal raphe nucleus in the control of REM sleep and wakefulness. GABA and Sleep 2010:189-98.
- [CrossRef] [Google Scholar]
- Electrical activity of the brain, sleep wake states, and circadian rhythms In: Ganong's Review of Medical Physiology, 26th editors. USA: McGraw-Hill Education; 2019. p. :618-47.
- [Google Scholar]






