Translate this page into:
Effect of Oral Glucose on Arterial Blood Gas and Oxygen Saturation on Exposure to Simulated Altitude of 12,000 Feet
Abstract
Aim:
To study the effects of oral intake of glucose on Arterial Blood Gas & Oxygen Saturation in Indian subjects on simulated altitude of 12,000’ in hypobaric chamber & to examine whether prior intake of oral glucose has any ameliorative effect on hypoxia tolerance.
Material & Methods:
20 Healthy male volunteers were subjected to moderate hypoxia at simulated altitude of 12,000’ in hypobaric chamber at the Department of High Altitude Physiology and Hyperbaric Medicine at IAM IAF after overnight fasting and 2nd time after one hour of ingestion of glucose (1gm/Kg body weight). Arterial Blood Gases were measured at ground as well as after 30 and 60 minutes of exposure. Vital parameters and End Tidal Carbon dioxide were also measured simultaneously.
Results:
Analysis of ABG parameters, ETCO2 and vital parameters did not bring out any effect of glucose ingestion on hypoxia induced by exposure to simulated altitude of 12,000’.
Conclusions:
No unequivocal changes were observed, on the analysis of ABG, SpO2 and ETCO2 parameters. The ventilation data in both conditions i.e. with or without glucose ingestion obtained from the respiratory rate also showed similar changes. As expected otherwise the results of the study did not support the speculation of better tolerance to hypoxia with ingestion of glucose.
Keywords
Arterial Blood Gases (ABG)
ETCO2
Hypoxia tolerance
Introduction
Acute hypobaric hypoxia has been frequently encountered and documented in aviation. It may be caused by ascent to high altitude while breathing air, failure of supplemental oxygen supply or loss of cabin pressurization. Symptoms of hypoxia depend upon the rate of ascent, altitude attained, duration at altitude and a host of other factors like temperature, physical fitness, acclimatization and individual variation in tolerance [1]. Besides supplemental oxygen, oxygen availability may also be increased by an increase in ventilation which is a natural compensatory mechanism observed during exposure to hypoxic environment. While an increase in ventilation at altitude is beneficial, it is associated with hypocapnia and resultant respiratory alkalosis, which in turn has profound physiological effects and makes it difficult to sustain the increased ventilatory effort. Increasing ventilation without inducing hypocapnia would thus be one of the preferential mechanisms for improving arterial oxygenation at altitude during short term exposure [2].
Carbon dioxide production at the cellular level is influenced by the metabolic substrate for energy production. In a subject consuming a typical mixed diet, Respiratory Exchange Ratio (RER) averages around 0.80 to 0.85. RER ranges from 0.71 when fat alone is being metabolized to 1.0 for a pure carbohydrate diet. When the body is in a constant or stable physiological state RER equals the metabolic respiratory quotient (RER = RQ) [3]. Therefore, it can be assumed that ingestion of high carbohydrate diet should increase carbon dioxide production in the body and the respiratory exchange ratio will be equal to or closer to 1 thus, increasing PAO2 as per the alveolar gas equation.Studies have proved that high carbohydrate diet leads to an increase in production of CO2 in the body. This in turn reduces the degree of hypocapnia following hypoxia induced hyperventilation and has an overall ameliorative effect on arterial oxygenation saturation and hypoxia tolerance. Hence, the use of high carbohydrate diets during acute hypoxic exposure has been suggested for many years now. Pugh proposed that carbohydrates can reduce the perception of altitude and that “the increased appetite for sugar at high altitude has a physiological justification in terms of respiration”. Subsequently, numerous investigators provided evidence that carbohydrate ingestion improves physical and mental tolerance to hypoxia in humans [4, 5, 6].
Thus, it is important to investigate whether carbohydrate ingestion may affect ventilation and consequently improve oxygen saturation of blood during acute hypoxic exposure. No study in the past has been carried out in this respect amongst Indian population. Therefore, the present study has been conceived to observe the effects of carbohydrate ingestion on hypoxia tolerance amongst Indian subjects in the controlled environment of the hypobaric chamber simulating acute hypoxic hypoxia.
Aim & Objectives
The aims were to study the effects of oral intake of glucose on Arterial Blood Gas (ABG) & Oxygen Saturation (SpO2) in Indian subjects on simulated altitude of 12,000 feet in hypobaric chamber. At the same time to examine whether prior intake of oral glucose has any ameliorative effect on hypoxia tolerance.
The objectives included measuring SpO2, End Tidal CO2 (ETCO2), Blood Pressure (BP), Heart Rate (HR), Respiratory Rate (RR) and ABG on ground and repeat it after reaching an altitude of 12,000 feet in hypobaric chamber after 30 min and 60 min of exposure.
Methodology
20 healthy male volunteers, between the age group of 20-45 yrs participated in the study. The participants acted as their own controls. All the subjects were ascertained to be healthy through history and clinical examination with special attention to ENT and detailed baseline investigations. The investigations included Hb%, TLC, DLC, Urine RE/ME, resting ECG, Pulmonary function test (PFT), Blood sugar – Fasting & Post prandial, HbA1c, Urea, Creatinine and Uric Acid. Apart from this, Body weight, Body mass index (BMI) and waist hip ratio (WHR) was also checked to rule out obesity.
Participants were instructed to have early and adequate sleep in the previous night. Subjects were asked to report fasting to the department at 0800h. This was done to reduce any confounding factors due to previous meal intake. Participants were also advised to avoid high fat and high carbohydrate diet in the previous night. A written informed consent was obtained from each of the participant.
Intra-arterial line was secured on both the days under strict aseptic conditions. The blood sample collected during the run was kept in ice buckets. They were demonstrated Valsalva maneuver to enable equalization of middle ear pressure if required, during the experiment. All the participants were asked to void bladder prior to the experiment. The protocol was approved by the Ethics Committee of the Institute of Aerospace Medicine.
Study Design
It was a within subject repeated observation cross over study. 20 participants were randomly divided into two groups, Test Group-I and Test Group-II. Each group consisted of 10 subjects. Both the test groups underwent hypoxia exposure at 12,000 feet on two occasions. First exposure was without glucose ingestion, and on second occasion with ingestion of glucose. For making it a cross over study test group-I was exposed to hypobaric hypoxia first without ingestion of oral glucose (Part-I experiment) and in the next exposure after ingestion of oral glucose (Part- II experiment), while test group-II were exposed to hypobaric hypoxia first with ingestion of glucose (Part-I experiment) and in the next exposure without ingestion of glucose (Part-II experiment).
Chamber Protocol
All the participants were given Ear Clearance Run (ECR) up to a simulated altitude of 10,000 feet prior to hypoxia exposure. The subjects were then taken to an altitude of 12,000 feet in the hypobaric chamber at an ascent rate of 3000 feet per minute (fpm) without oxygen. The participants were exposed to acute hypobaric hypoxia at 12,000 feet for 60 min. At the end of 60 minutes at 12,000 feet the chamber was brought down to ground level at a descent rate of 3,000 fpm.
Blood samples for ABG were drawn along with measurement of SpO2, ETCO2, BP, HR, RR, on ground and after reaching an altitude of 12,000 feet in hypobaric chamber twice, i.e. after 30 min and 60 min of exposure.
Results
The collected data was examined for normality using Shapiro-Wilk normality test. Student t test (one tailed, independent) was used assess the significance of cardiovascular and respiratory parameters recorded at ground and exposure at ‘0’, ‘30’ and ‘60’ minutes of exposure to hypobaric hypoxia at 12,000 feet, with and without glucose ingestion. Level of significance was accepted at p<0.05 with 95 % confidence limit. All participants in the study were males with ages ranging from 25 – 40 yrs (Table – 1 refers). The results of the experiment are presented in tables 2 - 9.

Discussion
Sausen et al [7], in a study conducted in 2001, found that the ETCO2 reduced on exposure to hypoxia. Earlier in 1988, Easton &Anthonisen found that ingestion of oral glucose in patients with Chronic Obstructive Pulmonary Disease (COPD) lead to a significantly higher VCO2, VO2, RQ and VE and reduced ETCO2 in comparison to controls [8]. This was also studied in comparison to a high fat diet [9]. Whether prior intake of oral glucose would have any ameliorative effect on hypoxia tolerance in healthy individuals by virtue of increased CO2 production as a result of cellular metabolism of glucose, presented a very basic query, which was considered worth exploring.
The main determinant of the difference in oxygen tension between inspired gas and alveolar gas on ascent to altitude is the alveolar CO2 tension. A fall in alveolar CO2 tension reduces the difference between the oxygen tensions in the inspired and alveolar gases. The tension of CO2 is determined by the ratio of CO2 produced to alveolar ventilation and this ratio is independent of environmental pressure [3].
The present study aimed to study the effects of oral intake of glucose on arterial blood gases & SpO2 in Indian subjects on acute exposure to hypoxic environment at 12,000 feet. PaO2, PaCO2, pH & bicarbonates were estimated from arterial blood samples during exposure to hypobaric hypoxia with and without administration of oral glucose in 20 healthy subjects in ages 30±3.45 (mean±SD, Range 25-35 yrs(Table -1 refers).
The effects of intake of oral glucose an hour prior to exposure to acute hypobaric hypoxia at 12,000 feet were examined. Special emphasis was given to rule out cases of metabolic abnormality, cardiovascular and respiratory diseases amongst the subjects which could have adverse impact on the outcome of the study. All the subjects tolerated the hypoxic stress well throughout the duration of test. Besides the vital parameters (including HR, RR & BP), arterial O2 saturation (SaO2), End Tidal CO2 (ETCO2) were measured at ground level and during acute exposure to hypoxic environment after 30 & 60 min. Blood samples from a secured IV Catheter were drawn at the same times for assessment of Arterial Blood Gas (ABG) analysis. The findings of the study in light of the extant literature are discussed in the succeeding paragraphs.
End Tidal Carbon Dioxide (ETCO2) and Respiratory Rate (RR)
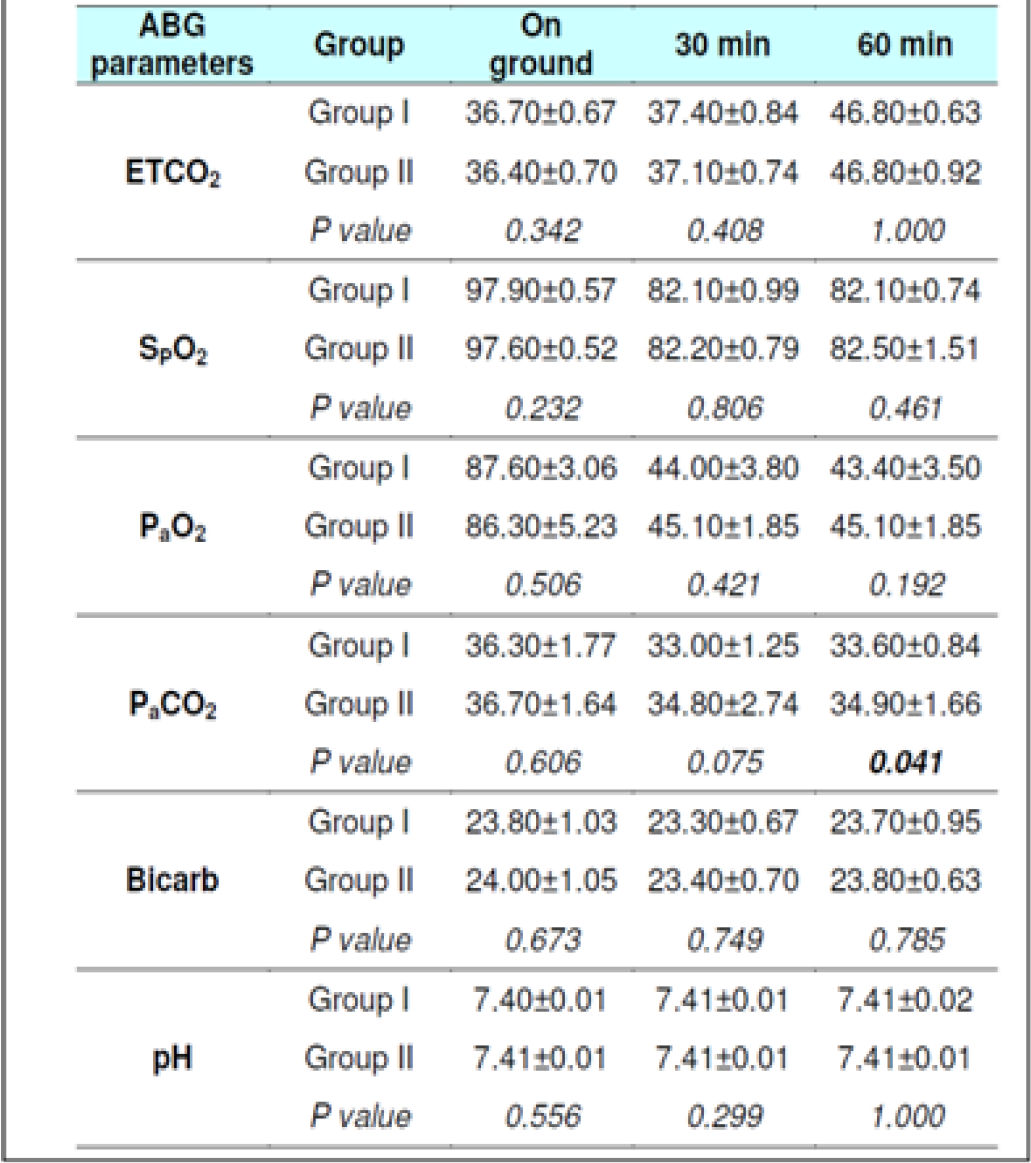
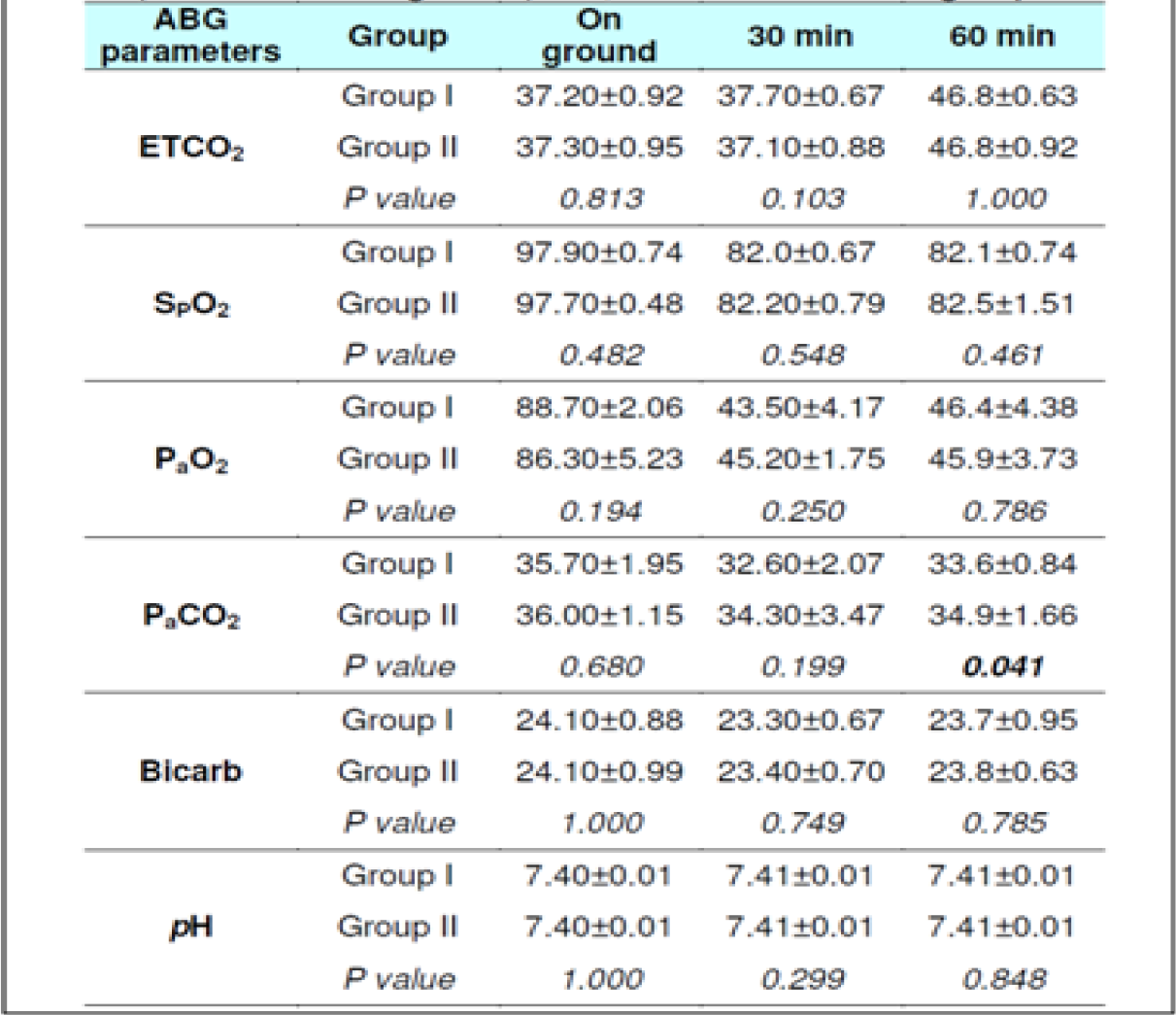
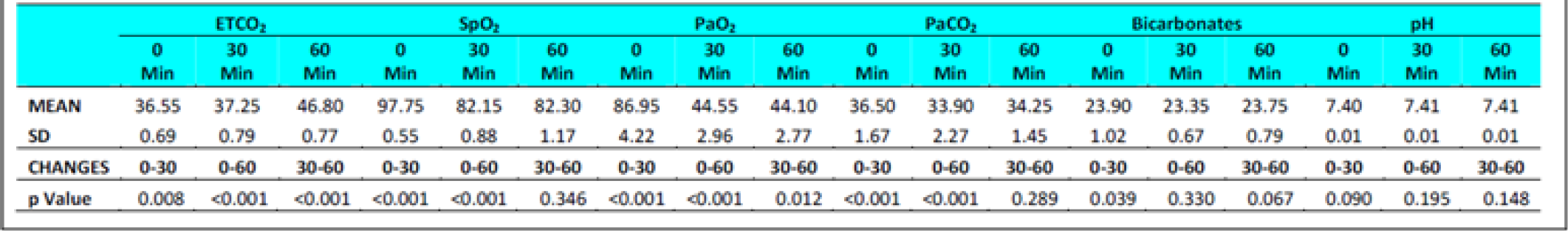
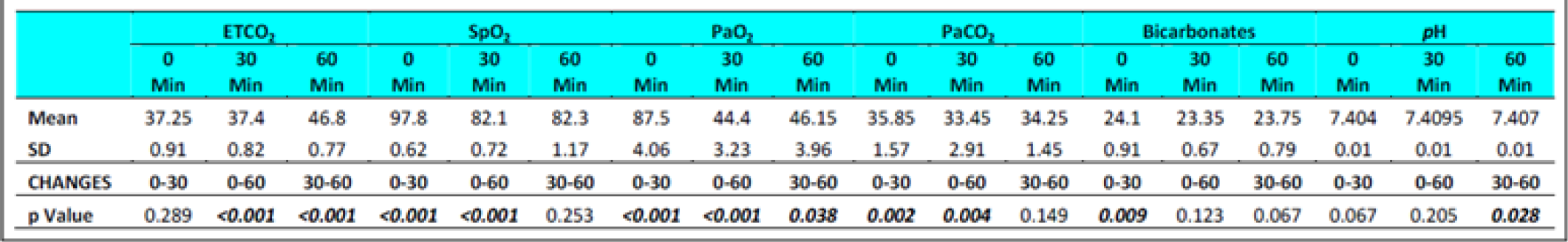
The ETCO2 level in fasting Group-I did not show any change from 0 to 30 min. However, in Group-II during fasting, an increase between 0 to 30 min (p = 0.044) was observed. The ETCO2 values in all subjects during fasting showed a significant difference between 0 to 60 min (p<0.0001) and 30 to 60 min (p<0.0001) in both the groups. Significant increase was also observed between 0 to 60 min (p<0.0001) and 30 to 60 min (p<0.0001) in both the groups when glucose was administered. There were no significant difference observed between the two groups with glucose administration (p = 0.278) between 0 to 30 min. This was not expected as the Respiratory rate across the subjects, and when group comparison was made, was found to be elevated significantly in both conditions ie with and without glucose from 0 to 30 min, 0-60 min and from 30-60 min. That is to say that while the ETCO2 showed a consistent increase, the RR showed a corresponding increase. This finding is against the expectations and what has been recorded in the literature. In a recent study carried out by Sahota in 2011 and earlier report by Ernsting (1965) [3, 10, 11], a consistent reduction in ETCO2 in subjects exposed to hypobaric hypoxia has been reported. While Ernsting measured changes within 10-20 min, Sahota reported changes in 90 minutes of exposure. In our study, the ETCO2 and RR (Table 4 and 7) were measured after 30 and 60 min to study the early effects of exposure with and without glucose.
Nancy et al studied subjects to a simulated altitude of 4600m (15,180 feet) with and without carbohydrate ingestion. They reported a decrease in PaCO2 from a mean of 36.4 ± 4.05 mm of Hg at the beginning of the study to 32.8 ± 4.41 after 150 min of hypoxia exposure in subjects who had not taken a carbohydrate rich solution, while in the group who had taken a carbohydrate rich solution the reduction after 150 min was to 33.9 ± 3.97 mm of Hg [12]. The increase observed was bare marginal after glucose ingestion and at the end of 150 min and hence the effects observed in the various studies can hardly be compared.
Hansen et al [13] exposed four subjects to a simulated altitude of 11,000 feet after either a high carbohydrate diet or low carbohydrate diet. The fall in PaCO2 was to an extent of 29.1 ± 2.9 mm of Hg in subjects when on low carbohydrate in comparison to a fall of PaCO2 to 30.4 ± 2.3 mm of Hg in high carbohydrate group. Similarly, Golja et al reported that CO2 fraction in the expired air increased significantly (p<0.001) in the carbohydrate intake group as compared with the control group who had not taken carbohydrate rich solution prior to exposure to acute hypobaric hypoxia [14]. The results of the present experiment showed a variable changes with initial decrease followed by increase but to lesser than the basal values (Table 6 and 7) this is not in agreement with the studies published earlier [3, 11, 12, 14].
Arterial Oxygen Saturation (SpO2)
The SpO2 level in fasting showed statistically significant difference between 0 to 30 min (p<0.001) & 0 to 60 min (p<0.001) in both the groups without glucose. Statistically significant difference was also observed between 0 to 60 min (p<0.0001) and 30 to 60 min (p<0.001) in both the groups when glucose was administered. There were no significant difference observed between the two groups with glucose administration (p = 0.288) as well as fasting state (p = 0.308) between 30 to 60 min.
Although the difference between the oxygen tension of the alveolar gas and that of the blood entering the pulmonary capillaries is markedly reduced on exposure to altitude, the diffusion characteristics of the alveolar-capillary membrane are such that the tension of oxygen leaving the pulmonary capillaries still equals that of alveolar gas when the individual is at rest. But the effects of reduction in atmospheric oxygen tension are evident through arterial oxygen tension (PaO2) and oxygen saturation of haemoglobin (SpO2). PaO2 decreases from 95 mm of Hg at ground level on breathing air to 56 mm of Hg at 8,000 feet, 43 mm of Hg at 12,000 feet and to 37 mm of Hg at 15,000 feet. Similarly, a comparable decrease in SpO2 from 97% at ground level to 93% at 8,000 feet on breathing air, 84% at 12,000 feet and 78% at 15,000 feet. So it can be derived that at a PaO2 of 43 mm of Hg at 12,000 feet the SpO2 is about 84% [3]. Hansen et al reported that at 11,000 feet of simulated hypoxia, subjects maintained PaO2 of 59.4 ± 5.8 mm of Hg with low carbohydrate diet while the same subjects maintained a PaO2 of 63.7 ± 3.0 mm of Hg after a high carbohydrate diet [13].
Nancy et al reported that both arterial oxygen tension and saturation trended upwards across most subjects from 45 min through 2 hours after carbohydrate ingestion, but only reached statistical significance at 60 min. Seven subjects ingested additional carbohydrates in the form of fruit juice 2 hours after the initial carbohydrate administration, these subjects maintained higher arterial oxygen compared to subjects who did not consume carbohydrates. This trend suggested additional effect with repeated carbohydrate intake [28]. Golja et al reported that in subjects without carbohydrate ingestion, SpO2 decreased from 99% to 86 % during exposure to hypoxia. In the Carbohydrate trial group SpO2 decreased from 99% to 90% in the comparable period of hypoxia exposure. The difference between the two trials was statistically significant (p < 0.001) throughout the hypoxic exposure [14]. Results of the present study on the decrease in SpO2 on exposure to acute hypobaric hypoxia and effects of carbohydrate ingestion are in agreement with previous reports [3, 12, 13, 14, 15].
Arterial Blood Gases (ABG) Parameters
The PaO2 level during fasting showed significant reduction between 0 to 30 min (p<0.001) & 0 to 60 min (p<0.001) in both the groups well as between 30 to 60 min in Group 2 (p = 0.041). Significant reduction was also observed when glucose was administered. The PaCO2 level in both fasting and post glucose administration showed significant reduction between 0 to 30 min & 0 to 60 min in both the groups as well as when analyzed across the subjects (Table 4,5 and 6,7). However, significant reduction was only observed between 0 to 60 min in both the groups and between 30 to 60 min (p = 0.008) in Group 1 when glucose was administered. The Bicarbonate level in fasting did not show any statistically significant difference between 0 to 30 min, 0 to 60 min and between 30 to 60 min in both the groups without glucose. Statistically significant difference was only observed between 0 to 30 min (p = 0.018) in Group 1 and when analysed across the subjects (p<0.009) when glucose was administered. The pH levels did not reveal any changes either between the groups or across all subjects at any stage with or without glucose (Table 4 and 7). No correlation between the parameters of ABG (Other than PaCO2) was observed with the ETCO2.
In 2012, Naidu analyzed the data of vast number of studies to see a correlation between PaCO2 and ETCO2 in neonates [16]. In analyzing the data presented, it became clear that whilst ETCO2 monitoring could not replace the Gold Standard of measuring PaCO2 it could provide useful information if used as a trending tool. This was further supported by studies by Rozycki et al, Wu et al, Bhat and Abhishek and Nangia et al from 1997 - 2003 [17, 18, 19, 20]. They found that a good correlation existed between ETCO2 and PaCO2 in patients without underlying lung disease. Haggerty et al [21] demonstrated a good degree of agreement by evaluating the use of the capnometer across two groups, they did not provide any confidence limits or intervals and a correlational relationship between ETCO2 and PaCO2 was not established. In the present study, no such correlation between ETCO2 and PaCO2 was found. The ABG parameters did not change significantly from fasting to post glucose administration in both the groups and across the subjects.
The gradient, or the difference between the PaCO2 and ETCO2 values, is a result of relationship between ventilation, airflow to the alveoli, and perfusion (referred to as V/Q or ventilation-perfusion matching or ratio). Calculating the gradient requires obtaining a simultaneous ABG sample and ETCO2. In normal healthy lungs there is a good match of alveolar ventilation and perfusion to the pulmonary capillaries resulting in ETCO2 that closely matches or correlates with the PaCO2. Therefore, PaCO2 may be estimated by using actual ETCO2 in normal subjects without any significant cardio-pulmonary disorders. The estimate is generally reliable if the ETCO2 trend is stable. In healthy subjects, the gradient is generally 2-5 mm Hg [22, 23]. In this study, however, a correlation between PaCO2 and ETCO2 was not observed.
Blood Pressure & Heart Rate changes
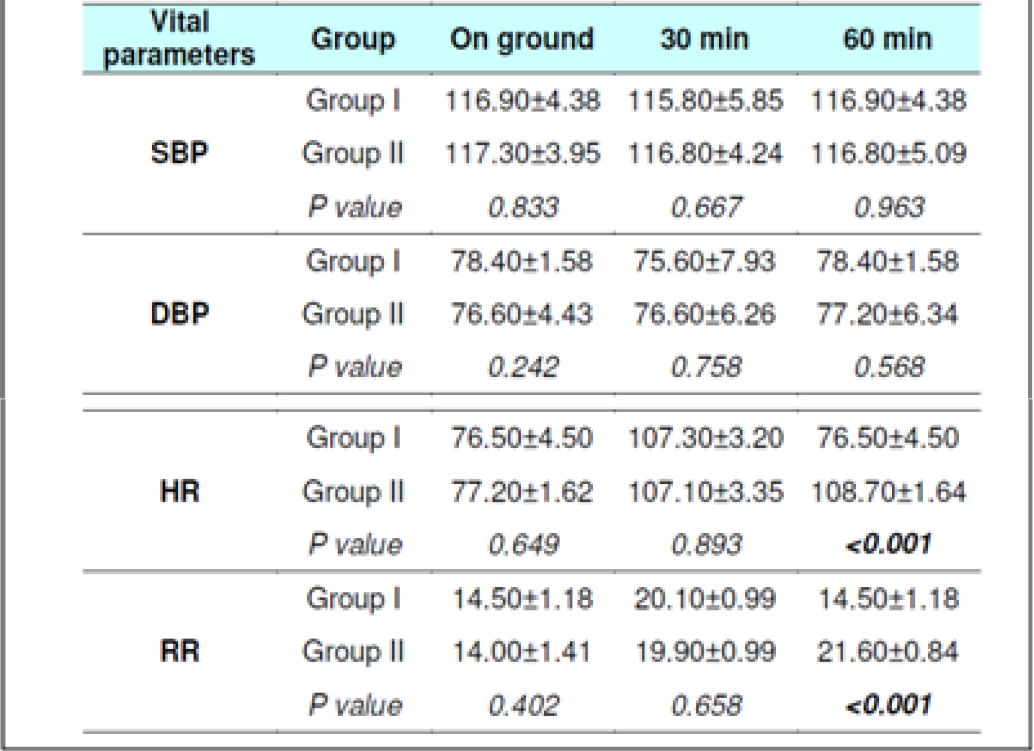
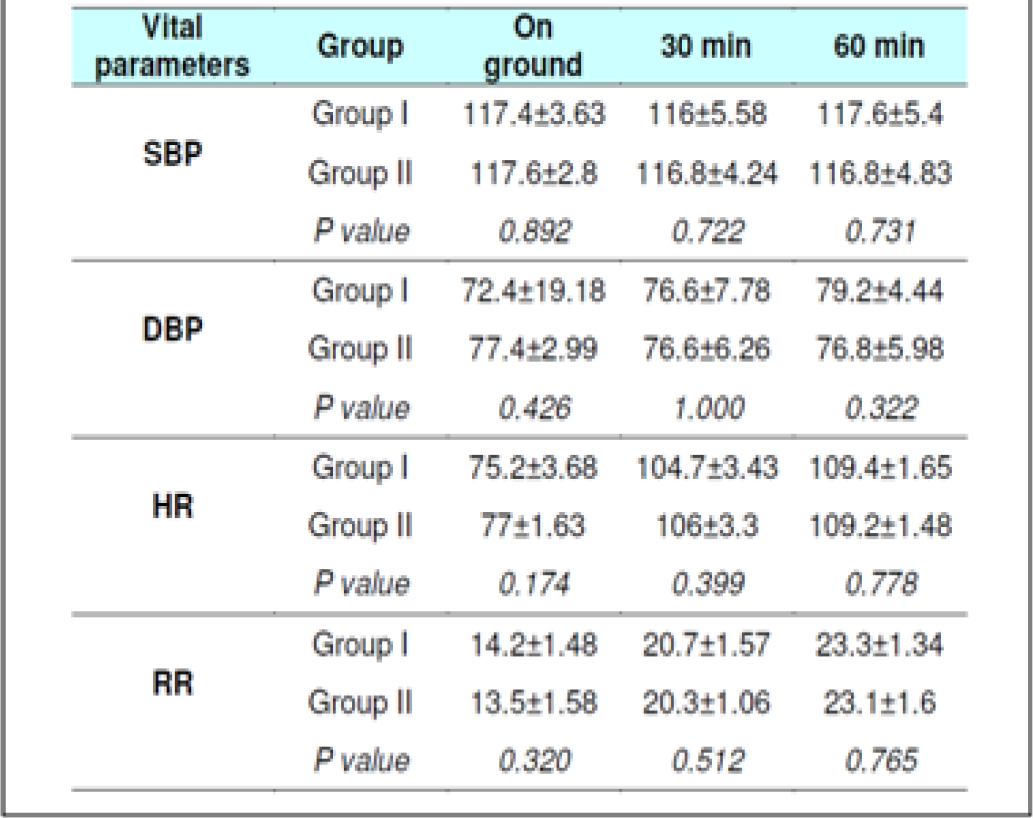
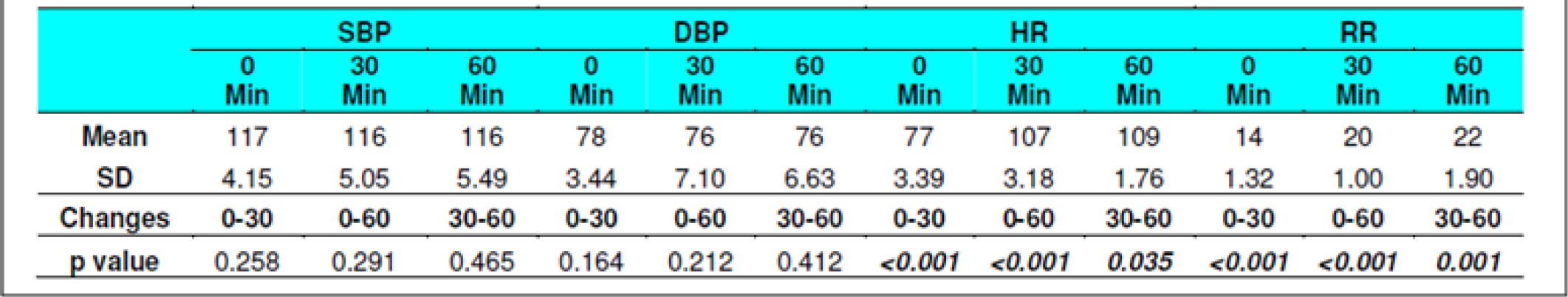
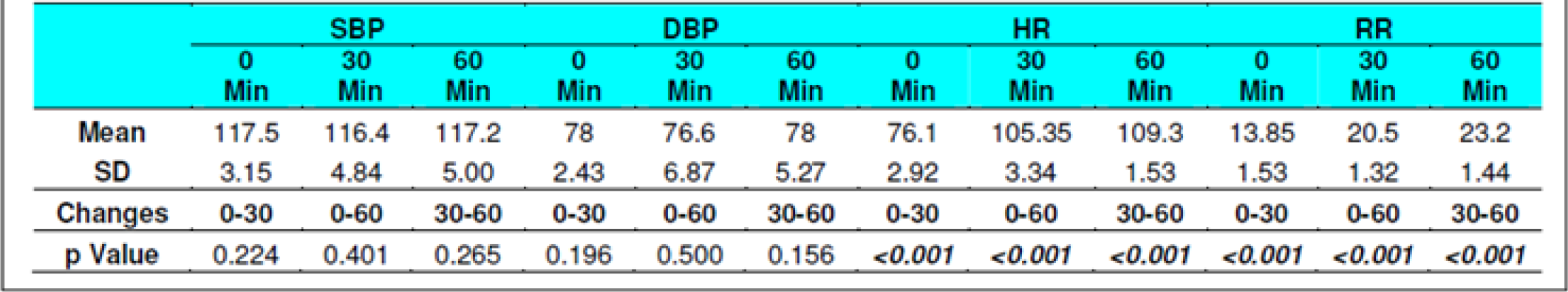
The Systolic Blood Pressure (SBP) and Diastolic Blood Pressure (DBP) level did not show any changes during the runs with or without glucose at any stage (Table 2, 3, 8 and 9). The results are in keeping with earlier published literature on effects of acute hypoxia on blood pressure. In a similar study, Golja et al reported that SBP remained statistically insignificant throughout the experiment [3, 14, 24, 25].
The heart rate increased significantly on exposure to hypoxia at 12,000 feet. The increase was highly significant (p<0.001) from 0 to 30 and 60 min both with and without glucose. The increase was also significant from 30 to 60 min in both the protocols. The Heart rate values did not vary significantly between the groups nor was any significant change seen from fasting to post glucose intake exposure across the subjects (Table 2, 3, 8 and 9). As a rule the mean arterial blood pressure does not change significantly during hypoxia from that of an individual breathing air at sea level [3, 24, 26]. The findings in this study are consistent with the findings of earlier researches. While in a similar study by Golja et al, HR was significantly elevated in the carbohydrate trial group compared to the control group without carbohydrate. The difference in HR between the two trials groups decreased toward the end of the experiment and did not reach statistical significance as is also seen in the present experiment [14].
In the study by Sahota [10] resting HR was significantly increased after ingestion of glucose than without glucose during the initial period of the experiment and remained higher throughout the experiment. In both the parts of the experiment that is with or without ingestion of glucose, the subjects were kept in a quiet environment at simulated altitude of 12,000 feet without indulging in any activity. But, the HR after ingestion carbohydrate in form of glucose was higher than without glucose. Berne et al. reported a small, but significant increase in HR following D-glucose ingestion and also observed an increase in sympathetic outflow in human muscle nerves in normoxia following carbohydrate ingestion, as determined by micro-neurography. Further, the results of Welle et al demonstrated that ingestion of carbohydrates, but not proteins or fat, increases plasma nor epinephrine level; this suggests that the activity in sympathetic nerve terminals is increased following carbohydrate ingestion. Similar results were also published by Young et al. It is yet to be answered as to whether this increase in increase in sympathetic activity is restricted to specific organs (heart, skeletal muscle) or whether it is a generalized response of the sympathetic nervous system to ingestion of carbohydrates [27, 28, 29]. Rowe et al suggested that hyperinsulinemia following carbohydrate ingestion results in a dose-dependent increase in sympathetic nervous system activity independent of changes in blood glucose. A hyper insulinimic response is expected after ingestion of glucose during the present study [30].
Whatever the mechanism of increase in heart rate it leads to increase in cardiac output without change in stroke volume and improves oxygen delivery during hypoxia more after ingestion of carbohydrates prior to hypoxia exposure [27, 28, 29].
Respiratory Responses to Hypoxia
The Respiratory Rate (RR) in fasting showed a significant increase from 14±1.32 to 20±1 and to 22± 1.9 (0 to 30 and 60 min without glucose – table 8 refers) similar increase was seen in both the groups without glucose as well as when glucose was administered. Eckman et al exposed 3 male subjects repeatedly to 15000 feet and 17000 feet for 1-2 hrs in an aircraft. They reported that minute ventilation following the protein meal was 7.0 per cent and 4.7 per cent less than the ventilations found with the carbohydrate meal or mixed diet. While, the average respiratory rate following a carbohydrate diet was 15.9 breaths per minute as compared to 14.9 and 14.4 breaths per minute in protein diet and mixed diet respectively [31]. Golja et al reported that minute inspiratory volume increased significantly in the carbohydrate trial as compared to the control trial [14]. The result of the present study is not commensurate with the observations of the above mentioned studies. Acute hypoxia leads to an increase in ventilation by increase in both rate and depth of breathing. In the present study, ventilation was collected in form of respiratory rate only which showed a significant increase in both the scenarios. While the above mentioned studies not only measured respiratory rate but also ventilation, volumes, concentration of oxygen and carbon dioxide in the expired air. This perhaps could be the reason for the difference in findings with the previous studies vis-à-vis the present one.
Conclusions
The present study examined the effect of carbohydrate ingestion on tolerance to moderate hypoxia (12,000 feet simulated altitude) of short term exposure (60 min) in 20 healthy male subjects in two experiment settings. Study was undertaken to study effect of glucose ingestion prior to exposure to moderate hypoxia exposure and whether it would have any effect on ABG parameters, arterial oxygen saturation and higher end tidal carbon di-oxide. No unequivocal changes were observed, on the analysis of ABG, SpO2 and ETCO2 parameters. The ventilation data in both conditions i.e. with or without glucose ingestion obtained from the respiratory rate also showed similar changes. As expected otherwise the results of the study did not support the speculation of better tolerance to hypoxia with ingestion of glucose. The studies in past have shown response which can be attributed to higher level of exposure and for longer duration. No studies in past have used ABG parameters in assessment of partial pressures of CO2 in alveoli or arterial blood probably owing to good correlation demonstrated between PaCO2 and ETCO2 . While a higher number of subjects exposed to increased durations and altitudes may bring out the differences with greater clarity, measurement of ABG parameters especially PaCO2, per se did not seem to add any additional information that might not be obtained by non-invasive measures of pulmonary ventilation, arterial oxygen saturation and end tidal CO2 estimation.
References
- Ventilatory response to hypoxia and carbon dioxide In: High altitude medicine and physiology. London UK: Arnold; 2000. p. :51-66.
- [Google Scholar]
- Ernsting’s Aviation and Space Medicine (5th ed). Florida (USA): CRC Press; 2016. p. :41-56.
- [Google Scholar]
- Pulmonary gas exchange In: Pulmonary physiology (7th ed). New York: The McGraw-Hill Companies Inc; 2007. p. :18-22.
- [Google Scholar]
- Gas transport between the lung & the tissues In: Review of medical physiology (22nd ed). New York: The McGraw-Hill Companies Inc; 2005. p. :666-78.
- [Google Scholar]
- Carotid body chemoreceptors: from natural stimuli to sensory discharges. Physiol Rev. 1994;74:829-98.
- [Google Scholar]
- The reduced oxygen breathing paradigm for hypoxic training: physiological, cognitive and subjective effects. Aviat Space Environ Med. 2001;72(6):539-45.
- [Google Scholar]
- Carbon dioxide effects on the ventilatory response to sustained hypoxia. J ApplPhysiol. 1988;64(4):1451-6.
- [Google Scholar]
- The effects of high fat and high carbohydrate diet loads on gas exchange and ventilation in COPD patients and normal subjects. Chest. 1993;104(1):189-96.
- [Google Scholar]
- To study the effects of oral intake of glucose on hypoxia olerance in Indian subjects. (dissertation). 2011;63
- [Google Scholar]
- A textbook of aviation physiology (1st ed). London: Pergamon Press; 1965. p. :214-261.
- [Google Scholar]
- Improvement in hypoxemia at 4600 meters of simulated altitude with carbohydrate ingestion. Aviat Space and Environ Med. 1999;70:874-78.
- [Google Scholar]
- Journal of App Physiol. 1972;33:441-45.
- [Google Scholar]
- Carbohydrate ingestion improves oxygen delivery in acute hypoxia. High Altitude Medicine and Biology. 2008;9:58-62.
- [Google Scholar]
- Comparative circulatory responses to systemic hypoxia in man and in unanesthetized dog. Journal of App Physiol. 1972;23(3):381-86.
- [Google Scholar]
- Is ETCO2 a predictor of PaCO2 in ventilated neonates on the Neonatal Intensive Care Unit? Working Papers in Health Sciences. ;1:1. [internet]. [Cited 2018 Jan 23]. Availablefrom: https://pdfs.semanticscholar.org/34aa/eb7e5a667f311fbd8bd792a6ff717a8c35b7.pdf
- [Google Scholar]
- Mainstream end-tidal carbon dioxide monitoring in the neonatal intensive care unit. Paediatrics. 1998;101(4):648-53.
- [Google Scholar]
- Good estimation of arterial carbon dioxide by end-tidal carbon dioxide monitoring in the neonatal intensive care unit. Pediatric Pulmonology. 2003;35(4):292-95.
- [Google Scholar]
- Mainstream end-tidal carbon dioxide monitoring in ventilated neonates. Singapore Medical Journal. 2008;49(3):199-203.
- [Google Scholar]
- End tidal carbon dioxide monitoring-Its reliability in neonates. Indian Journal of Paediatrics. 1997;64:389-94.
- [Google Scholar]
- Accuracy of a new low-flow side stream capnography technology in newborns. Journal of Perinatology. 2002;22(3):219-25.
- [Google Scholar]
- [Cited 2014 Oct 20] Available from: http://www.ld99.com/reference/old/text/2878909-516.html
- [Google Scholar]
- [Cited 2014 Oct 20] Available from: http://www.procamed.ch/pdf/etco2_gradient.pdf
- [Google Scholar]
- ID Green In: Textbook of Aviation Physiology (1st ed). London: Pergamon Press; 1965. p. :264-69.
- [Google Scholar]
- Venous pressure and circulation time during acute progressive anoxia in man. Amer J of physiol. 1943;138:593-98.
- [Google Scholar]
- The effect of hypoxemia on ventilation and circulation in man. Amer J of Physiol. 1941;132:426-36.
- [Google Scholar]
- Sympathetic response to oral carbohydrate administration: evidence from microelectrode nerve recordings. J Clin. Invest. 1989;84:1403-09.
- [Google Scholar]
- Thermic effect of feeding in man: increased plasma norepinephrine levels following glucose but not protein or fat consumption. Metabolism. 1981;30(10):953-58.
- [Google Scholar]
- Stimulation of the sympathetic nervous system during sucrose feeding. Nature. 1977;269:615-61.
- [Google Scholar]
- Effect of insulin and glucose infusions on sympathetic nervous system activity in normal man. Diabetes. 1981;30:219-25.
- [Google Scholar]






