Translate this page into:
Combined altitude depleted oxygen vis-à-vis hypobaric hypoxia: Efficacy in hypoxia indoctrination

*Corresponding author: Sanjay Purushothaman, Training Wing, Institute of Aerospace Medicine, Bengaluru, Karnataka, India. sanjaypurushothaman@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Purushothaman S, Sarkar R, Joshi VV, Ningaiah M. Combined altitude depleted oxygen vis-à-vis hypobaric hypoxia: Efficacy in hypoxia indoctrination. Indian J Aerosp Med 2022;66:57-64.
Abstract
Introduction:
The Combined Altitude and Depleted Oxygen (CADO), as an alternate method for hypoxia indoctrination, has the dual advantages of exposing to an altitude less than the threshold for decompression sickness, a known risk in training using Hypobaric Hypoxia (HH) while accounting for the pressure changes due to altitude, a limitation of using normobaric hypoxia.
Objectives:
This study aimed to evaluate the efficacy of CADO in hypoxia indoctrination by comparing it with the time-tested gold standard method of HH.
Material and Methods:
Forty subjects were exposed to both CADO and HH, simulating 25,000 ft for a maximum period of 5 min. CADO was achieved by combining exposure to an altitude of 10,000 ft in the hypobaric chamber and breathing a hypoxic gas mixture of 10.3% oxygen and 89.7% nitrogen. Physiological parameters (oxygen saturation, heart rate, and respiratory rate) and psychomotor performance (dual task test component of pSuMEDhA) were compared between the two exposures. The incidence and severity of subjective symptoms were also compared at the end of exposures.
Results:
No significant difference was observed in the physiological parameters and psychomotor performance during the two exposures. Out of the 24 common symptoms of hypoxia assessed, there was a higher incidence of 20 symptoms in subjects exposed to HH compared to CADO. The severity of 15 symptoms was also found to be significantly greater (P < 0.05) in subjects exposed to HH.
Conclusion:
The similarity of physiological changes in CADO and HH shows the potential application of CADO as a tool for hypoxia demonstration. However, in view of decreased incidence and severity of subjective symptoms in CADO compared to HH, CADO cannot be considered equivalent to the gold standard (HH) for hypoxia indoctrination for high-risk individuals, namely, aircrew and combat free fall personnel. CADO as a modality can be used as a tool for hypoxia demonstration for persons not involved in flying duties and for high-altitude research.
Keywords
Hypoxia indoctrination
Combined altitude depleted oxygen
Hypobaric hypoxia
Normobaric hypoxia
Dual task test
PSuMEDhA
INTRODUCTION
Exposure to hypoxia results in the appearance of a variety of symptoms, which may vary from individual to individual in terms of their speed and order of appearance as well as severity. However, there is considerable consistency in the symptom complex experienced by a particular individual on repetitive exposure to acute hypoxia.[1,2] This consistency in an individual’s response to acute hypoxia is termed as “hypoxia signature,” as described by Smith[3] and this forms the basis of hypoxia awareness training imparted to aircrew and other high-risk individuals such as Combat Free Fall (CFF) personnel around the world. The principal element of hypoxia indoctrination is the intentional eliciting of hypoxia symptomatology during training sessions within a safe and controlled environment to enable aircrew to learn and recognize the danger posed by the in-flight onset of hypoxia.
The training has been traditionally carried out with the help of hypobaric altitude chambers simulating a physiological altitude of 25,000 ft. The overall baseline risk of Decompression Sickness (DCS) during hypoxia awareness training using Hypobaric Hypoxia (HH) was estimated to be 1/1000 for altitude exposure above 18,000 ft in a study by Dully in 1992.[4] To mitigate this risk, normobaric oxygen dilution techniques (Reduced Oxygen Breathing Device) were developed. Normobaric Hypoxia (NH) while being safer is also considered to have greater fidelity in the training of fast jet aircrew as it simulates “mask on” hypoxia. However, the major disadvantage of the NH method is that it completely eliminates the pressure changes induced by altitude exposure and lacks realistic perception.
Recently, an alternate training method was developed by the Royal Australian Air Force Institute of Aviation Medicine (RAAF AVMED),[5] which accounts for the lack of pressure changes seen in NH, while exposing the subjects to an altitude less than the threshold for DCS. This method referred to as Combined Altitude and Depleted Oxygen (CADO) combines exposure to a physiologically safer altitude in the hypobaric chamber while breathing a hypoxic gas mixture to simulate a physiological altitude of 25,000 ft. As of now, there is only one study[5] comparing the efficacy of hypoxia training imparted by CADO, which found that CADO compared well with HH. However, studies have shown that hypobaria itself has a definitive effect on the physiological response[6] as well as inducing symptoms of hypoxia[7] and that the HH environment is more stressful when compared to NH. Numerous other studies have been sceptical about considering NH to be equivalent to HH.[8-11] CADO is unique in that it combines both hypobaric and hypoxic elements to induce hypoxia in a subject.
This present study aimed to compare CADO with the time-tested gold standard method of HH in imparting hypoxia awareness training in our set up. The objective was to collect, analyze, and compare subjective and objective data from the same cohort of subjects during exposure to HH and CADO. The subjective data comprised a record of signs and symptoms experienced by the subjects, while the objective data comprised the physiological parameters as well as psychomotor performance assessment during each exposure.
MATERIAL AND METHODS
Subjects
Forty healthy volunteers (38 men and two women) with a mean age of 31.78 ± 4.78 years participated in this study. The inclusion criterion was healthy subjects between the ages of 20–40 years. Those with a history of cardiac or respiratory illness, illness in the sinuses and ears, blood donation or scuba diving in the previous week, as well as those who had flown above 10,000 ft (3048 m) in the previous 24 h and those who lived at high altitudes (1000 m) were excluded from the study. The subjects were instructed to refrain from alcohol for at least 12 h before study and refrain from smoking for at least 2 h before the study.
Materials
Hypobaric chamber
HH equivalent to the desired altitude of 25,000 ft was simulated in the Explosive Decompression Chamber (EDC) at the Department of High-Altitude Physiology and Hyperbaric Medicine, Institute of Aerospace Medicine, Indian Air Force.
CADO
CADO simulating a physiological altitude of 25,000 ft was administered by combining exposure to 10,000 ft in the EDC while breathing a hypoxic gas mixture containing 10.3% oxygen and 89.7% nitrogen. This hypoxic mixture was delivered to the subjects with the help of portable lightweight cylinders. This mixture containing 10.3% oxygen was selected because breathing an Inspired Fraction of Oxygen (FiO2) of approximately 10% at 10,000 ft (3048 m) would give an Inspired Partial Pressure of Oxygen (PiO2) equivalent to breathing air at 25,000 ft (7620 m). This was calculated using the Equivalent Air Altitude (EAA) model based on Dalton’s law of partial pressures and correcting for water vapor pressure (47 mmHg at 37°C) using the equation PiO2 = FiO2 (PB - 47). Here, PiO2 is 49 mmHg, and barometric pressure (PB) is 523 mmHg (at 10,000 ft), hence FiO2 to simulate 25000 ft while at 10,000 ft would be FiO2 = 49 ÷ (523-47) = 0.103, or 10.3%.[5]
Equivital wireless physiological monitoring system
This was used to measure the physiological parameters; Peripheral Oxygen Saturation (SpO2), Heart Rate (HR), and Respiratory Rate (RR). The system consists of a compact and unobtrusive sensor belt, a data logger in the form of a sensor electronics module, and a wired ancillary to record SpO2.
Psychomotor performance task
Psychomotor performance was assessed with the help of a Dual Task Test (DTT), which is a component of an indigenously developed cognitive test battery “pSuMEDhA.” The DTT is a psychomotor test which assesses the cognitive faculties of tracking, pursuit, and psychomotor coordination. The test consists of two simultaneous tasks. A yellow ball moves around the screen which needs to be tracked by the participant with a mouse. In addition to this, a change in color of the ball to RED will require the participant to react with a key press. The average duration of the test is 03 min. The distance of the pointer from the center of the ball will be measured every five milliseconds and the average deviation (lag error) of the center of the ball to the mouse pointer will be calculated and expressed in pixels.[12] The DTT report consists of five output parameters: Response Time (RT) (in milliseconds), correct clicks, Extra Clicks (EC), Missed Clicks (MC), and Lag Error (LE) (pixels). The subjects performed DTT during the first 03 min of the 05 min hypoxia exposures [Figure 1].

- Subject performing dual task test during combined altitude and depleted oxygen.
Hypoxia symptom questionnaire
Subjects were asked to mark the symptoms that they had experienced by filling the hypoxia symptom questionnaire which included the 24 commonly reported symptoms of hypoxia. This represented the incidence of symptoms. They also marked the severity of each symptom experienced on a 10 cm linear visual analog scale ranging from “nil” to “extreme,” which was then converted to a digital scale of 0–10 by simple linear measurement. This represented the symptom severity score.
Study protocol
The study protocol was approved by the Institute Ethics Committee. The subjects were briefed on the study protocol and written informed consent was obtained. On the day of experimentation, baseline data of RR, HR, and peripheral SpO2 were collected for a period of 05 min with the help of the equivital physiological monitoring system. The subjects also performed the DTT at the ground which served as the baseline values for the various parameters evaluated by the DTT.
The subjects were exposed to both types of hypoxia (CADO and HH) with each hypoxia session separated by a period of at least 24 h to rule out a carry-over effect. The order of the hypoxia exposures was randomized. The maximum duration of exposure for both the hypoxia sessions was set at 5 min as the time of useful consciousness at 25,000 ft as 270 ± 96 s.[1]
In the HH exposure, the subjects pre-breathed 100% oxygen for 30 min before the altitude exposure to protect against DCS. In both the hypoxia exposures, the subjects underwent the ear clearance run first, where they were taken to a simulated altitude of 10,000 ft at the rate of 3000 ft/min and brought back down to ground level again at 3000 ft/min. This test was conducted to assess the patency of eustachian tube.
In the CADO exposure, the subjects were taken to 10,000 ft in the EDC, and then, they were made to breathe the hypoxic gas mixture containing 10.3% oxygen and 89.7% nitrogen for 05 min. In the HH exposure, the subjects were taken to 25,000 ft in the EDC at the rate of 3000 ft/min. The subjects continued to breathe 100% oxygen until they reached 25,000 ft. At the target altitude of 25000 ft, the subjects were asked to remove the mask for 05 min. The subjects performed the DTT during the first 03 min of both the exposures. The criteria for completion of exposure were either stipulated 5 min of exposure or a fall in SpO2 below 65% or appearance of debilitating symptoms. After completing the hypoxia exposures, the subjects filled out the hypoxia symptom questionnaire.
Statistical analysis
All the parameters assessed were compared between three groups (CADO, HH, and Baseline) except for subjective symptoms which were compared only between CADO and HH. The statistical analysis was carried out using SPSS 20. The level of significance was kept at P < 0.05.
RESULTS
All 40 subjects completed at least 03 min of both the exposures (CADO and HH). For the CADO exposure, 31 subjects completed 04 min, while only 24 were able to complete the entire 05 min. For HH, 31 subjects completed 4 min of the exposure while only 18 were able to complete the entire 5 min.
Physiological parameters
The mean values of SpO2, HR, and RR at the end of each minute of the exposures for both CADO and HH were tabulated as T1, T2, T3, T4, and T5 (5 time intervals).
Peripheral SpO2
The mean baseline SpO2 of the 40 subjects was 96.63 ± 0.95%. The mean SpO2 at the end of each minute of the exposures for both CADO and HH is represented in Figure 2 (including Baseline value). The minimum mean values for SpO2 during CADO and HH were 67.85 ± 5.01% at T3 and 66.68 ± 3.64% at T4, respectively. ANalysis Of VAriance (ANOVA) showed that mean SpO2 readings differed significantly between the three groups (CADO, HH and Baseline) at all 5 time intervals (P = 0.000). A post hoc analysis showed that during both CADO and HH, there was statistically significant reduction in mean SpO2 at all 5 time intervals compared to the baseline (P = 0.000). Post hoc comparison between the mean SpO2 at the end of each minute between the CADO and HH was not significantly different at all 5 time intervals.
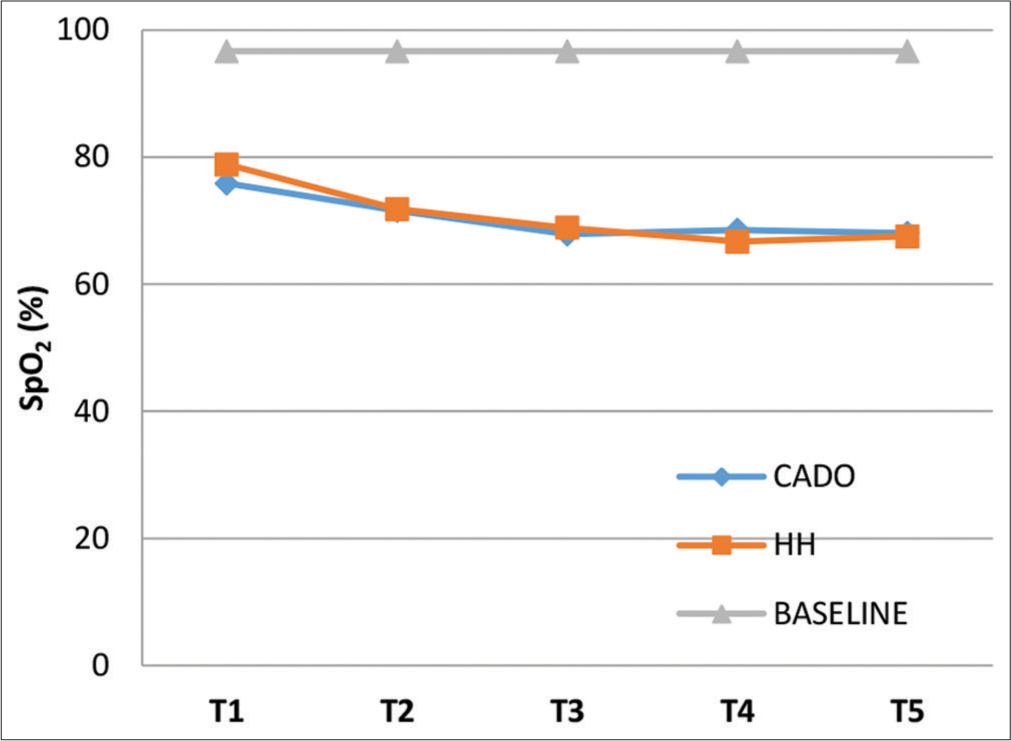
- Graph showing mean SpO2 at T1, T2, T3, T4, and T5 for the three groups.
HR
The mean baseline HR was 77 ± 7.2 bpm. The mean HR at the end of each minute of the exposures for both CADO and HH are represented in Figure 3 (including Baseline value). ANOVA showed that mean HR readings differed significantly between the three groups (CADO, HH, and baseline) at all 5 time intervals (P = 0.000). The maximum mean values for HR during CADO and HH were 103.18 ± 11.63 bpm at T3 and 108.67 ± 13.47 bpm at T5, respectively. A post hoc analysis showed that during both CADO and HH, there was statistically significant increase in mean HR at all 5 time intervals compared to the baseline (P = 0.000). Post hoc comparison between mean HR was not significantly different between the CADO and HH exposures at all 5 time intervals except T4. At T4, the mean HR of HH was significantly greater than the mean HR of CADO with a mean difference of 6.90 bpm (P = 0.025), which may be due to subjects aborting the test in CADO due to low SpO2 in the 4th min, which also explains the apparent fall in mean HR from T3 to T4 during CADO.
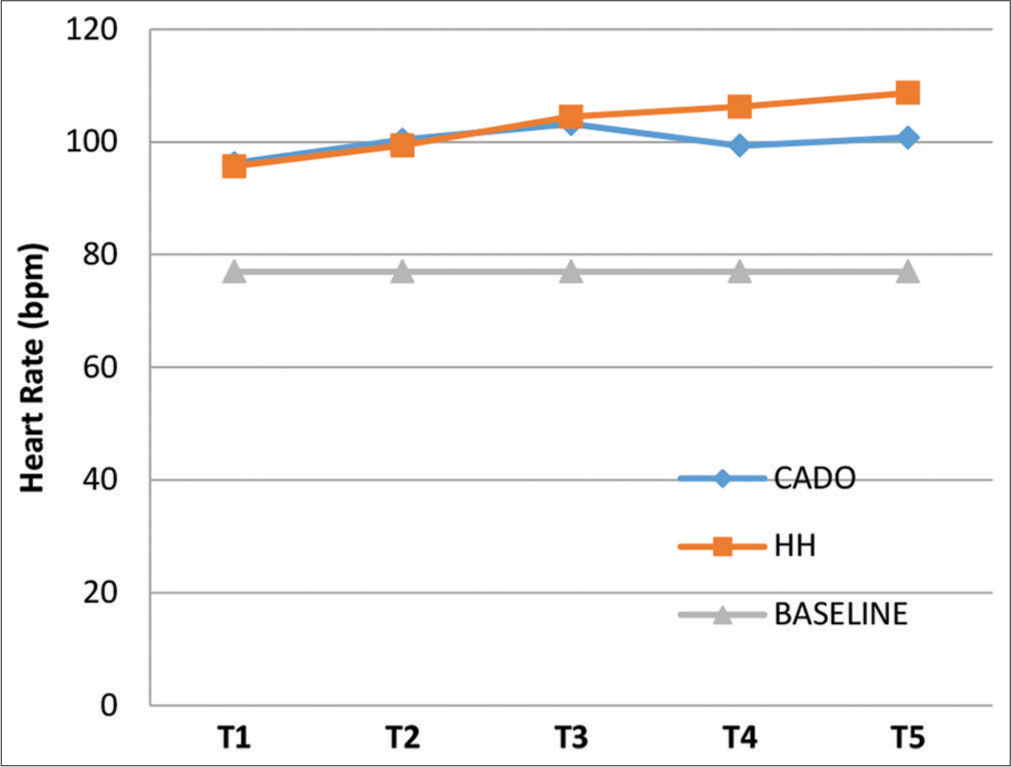
- Graph showing mean heart rate at T1, T2, T3, T4, and T5 for the three groups.
RR
The baseline RR was 13.13 ± 1.16 breaths/min. The mean RR at the end of each minute of the exposures for both CADO and HH is depicted in Figure 4 (including Baseline value). The maximum mean values for RR during CADO and HH were 19.13 ± 4.72 breaths/min at T5 and 19.74 ± 3.44 breaths/min at T4, respectively. An ANOVA showed that mean RR readings differed significantly between the three groups (CADO, HH, and baseline) at all 5 time intervals (P = 0.000). A post hoc analysis showed that during both CADO and HH, there was statistically significant increase in mean RR at all 5 time intervals compared to the baseline (P = 0.000). Post hoc comparison between mean RR was not significantly different between the CADO and HH exposures at all 5 time intervals. The apparent fall in mean RR values from T3 to T4 during CADO and T4 to T5 in HH can be attributed to subjects dropping out due to low SpO2.
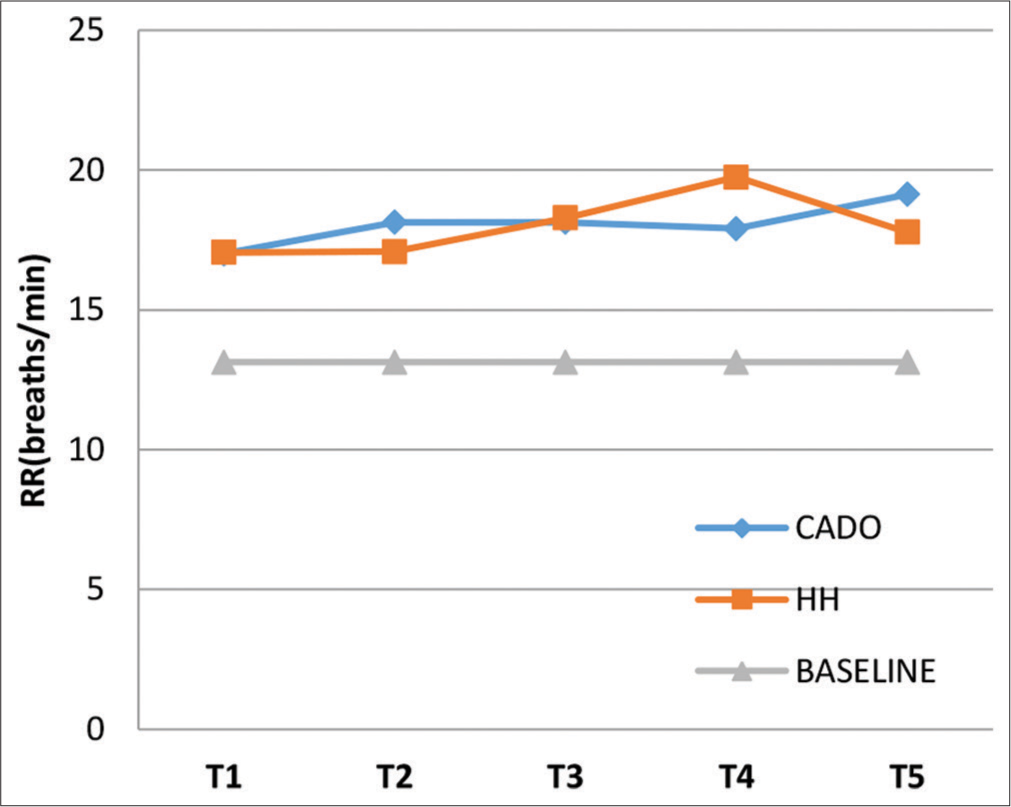
- Graph showing mean respiratory rate at T1, T2, T3, T4, and T5 for the three groups.
Psychomotor performance (DTT)
The output of the DTT is in terms of RT, (CC), EC, MC, and LE. An ANOVA showed that the means of three parameters of the DTT (RT, MC, and LE) differed significantly between the three groups (CADO, HH, and baseline), while there was no significant difference between the means of CC and EC.
A post hoc analysis showed a significantly higher mean RT for CADO and HH compared to baseline RT with a mean difference of 56.03 ms and 56.10 ms, respectively, with P value of 0.000 for both. There was no significant difference between mean RT of CADO and HH. For Correct Clicks (CC), the only significant difference was found between the means of CC at HH and baseline with P value of 0.026 and a mean difference of 0.90. There was no significant difference found between CADO and baseline as well as between CADO and HH. For EC, there was no significant difference found between any of the groups. For MC, the only significant difference was between the means of MC at HH and baseline with P value of 0.018 and a mean difference of 0.40. There was no significant difference found between CADO and baseline as well as between CADO and HH. The mean LE for CADO and HH was found to be significantly different from baseline LE with a mean difference of 2.40 pixels (P = 0.000) and 1.66 pixels (P = 0.008). There was no significant difference between mean LE of CADO and HH.
Subjective symptoms
Incidence
The incidence of hypoxia symptoms Figure 5 was equal between the two exposures for symptoms of faint, headache, and irritable. Warm was the only symptom which had a higher frequency in CADO compared to HH. All the other symptoms had a higher incidence in HH. The three most common symptoms in CADO were short of breath, thinking slow, and light headedness. The three most common symptoms in HH were thinking slow, making mistakes, and reaction slow.

- Incidence of symptoms during combined altitude and depleted oxygen and hypobaric hypoxia.
Severity
The mean severity for each of the symptoms during the two exposures on a 10-point scale is tabulated at Table 1. The three most severe symptoms in CADO were short of breath, thinking slow, and reaction slow, whereas those in HH were thinking slow, reaction slow, and light headed. A paired two-tailed t-test revealed a statistically significant difference in mean severity score between CADO and HH in 15 symptoms with all these symptoms having higher severity in HH.
| Symptom | Mean severity score |
Sig. | |
|---|---|---|---|
| CADO | HH | ||
| Light headed | 2.675 | 3.825 | 0.001 |
| Reaction slow | 2.95 | 4.15 | 0.003 |
| Thinking slow | 3 | 4.225 | 0.002 |
| Concentration off | 2.575 | 3.8 | 0.009 |
| Coordination off | 2.35 | 3.25 | 0.039 |
| Dizzy | 2.4 | 3.25 | 0.049 |
| Faint | 1.975 | 2.125 | 0.716 |
| Warm | 2.5 | 2.1 | 0.256 |
| Making mistakes | 2.925 | 3.7 | 0.049 |
| Numbness | 1.425 | 2.4 | 0.003 |
| Mentally tired | 1.95 | 3.125 | 0.010 |
| Tingling | 1.425 | 2.175 | 0.038 |
| Hand shaking | 1.525 | 2.375 | 0.024 |
| Heart pounding | 2.05 | 2.475 | 0.208 |
| Vision dim | 1.4 | 2.05 | 0.028 |
| Short of breath | 3 | 3.275 | 0.445 |
| Weak | 1.85 | 2.35 | 0.185 |
| Euphoric | 1.475 | 1.95 | 0.005 |
| Physically tired | 1.55 | 2.1 | 0.024 |
| Nervous | 1.9 | 2.45 | 0.026 |
| Sleepy | 1.775 | 2.15 | 0.256 |
| Restless | 1.875 | 2.525 | 0.077 |
| Headache | 1.55 | 1.55 | 1.000 |
| Irritable | 1.625 | 1.875 | 0.394 |
CADO: Combined altitude and depleted oxygen, HH: Hypobaric hypoxia P value significant (<0.05)
DISCUSSION
Hypoxia remains a significant threat for aircrew. Hypoxia awareness training is a vital cog in ensuring flight safety as it induces hypoxia in aircrew in a controlled environment where they can appreciate their individual symptoms. This helps to recognize the hypoxia symptoms in the future, and thus, the aircrew can take appropriate countermeasures to prevent in-flight hypoxia. While both the hypobaric chamber and normobaric oxygen dilution techniques have been used in hypoxia awareness training around the world, the RAAF AVMED developed CADO in 2011, which combines both the elements of hypobaric and NH to simulate a physiological altitude of 25,000 ft. The literature is scant on comparative studies between CADO and HH. The present study aimed to compare CADO, achieved by combining exposure to 10,000 ft in the hypobaric chamber while breathing a hypoxic gas mixture containing 10.3% oxygen and 89.7% nitrogen with the time-tested gold standard method of HH in imparting hypoxia awareness training in our set up.
There was a significant reduction in mean SpO2 during CADO and HH that were similar between the two exposures. The fall in SpO2 in both exposures can be explained by the fact that on acute exposure to hypoxia irrespective of the method, the PiO2 in the air, alveoli, and blood is reduced, and oxyhemoglobin desaturates, reducing the arterial oxygen content.[13,14] The findings of this study are in accordance with the study by Singh et al. That compared the fall in mean SpO2 during CADO and HH.[5] The findings are also in congruence with studies by Sausen et al. which looked at a fall in SpO2 on exposure to NH using ROBD.[6,15] Comparative studies on the effectiveness of HH and NH in eliciting changes in SpO2 also showed similar results.[7,16-18]
The rise in mean HR during exposure to hypoxic conditions is a known entity. This is due to sympathetic activation directly through stimulation of peripheral chemoreceptor and vagal withdrawal indirectly through increases in ventilation, resulting ultimately in tachycardia and an increase in cardiac output.[13] In the present study, there was a similar rise in mean HR during CADO and HH. This finding is in concordance with the study by Singh et al. comparing the rise in mean HR between CADO and HH.[5] Comparative studies on the effectiveness of HH and NH using ROBD in eliciting significant changes in HR by Kumar et al. and Faiss et al. showed similar results.[16,19]
The rise in RR as an acute response to hypoxia is the net result of various feedback mechanisms which constitute the hypoxic ventilatory response. The main driving mechanism in increasing ventilation is raised neural output from peripheral chemoreceptors, mainly carotid bodies which principally sense hypoxia.[20] In this study, the rise in mean RR during CADO was similar to that seen in HH. The study by Singh et al. comparing CADO with HH[5] did not talk about changes in ventilation during the exposures. However, comparative studies between HH and NH using ROBD by Faiss et al. and Loeppky et al. showed similar results.[19,21] Thus, CADO compared well with other forms of hypoxia exposures in eliciting changes in RR for the purpose of hypoxia awareness training.
In this study, there was similar psychomotor performance impairment during both the hypoxia exposures of CADO and HH in the form of increased RT and increased LE, while performing the DTT. The psychomotor performance impairment seen in this study is in accordance with a study by Dart et al.[22] which found immediate performance decrements in the form of increased RTs on exposure to an altitude of 20,000 ft. A study by Phillips et al.[23] also showed similar psychomotor performance impairment in the form of increased two-choice reaction time on exposure to a hypoxic air mixture simulating an altitude of 20,000 ft. The psychomotor impairment seen in this study also compares well with other studies which assessed cognitive functioning during NH exposures.[6,15,16] The finding of increased RTs in the present study is in agreement with the study by Singh et al which compared psychomotor performance between CADO and HH.[5]
The incidence and severity of symptoms in HH were observed to be much more compared to CADO in contrast to the study by Singh et al.[5] However, our findings are in accordance with a study by Roach et al.,[7] which found that the incidence of Acute Mountain Sickness (AMS) was more when subjects were exposed to HH compared to NH simulating the same altitude. This was explained by sodium and fluid retention in humans which were attributed to effects of hypobaria itself without hypoxia, which was also brought out in another study by Epstein and Saruta.[24] Hypobaria has also been found to increase the blood–brain barrier permeability in rabbits[25] and Roach et al.[7] proposed that the interaction of hypobaria and hypoxia results in the increased incidence of AMS in HH compared to NH. Another study by Loeppky et al.[18] also attributed the higher incidence of AMS in HH to fluid retention and elevated anti-diuretic hormone levels found on exposure to HH. In addition, Conkin and Wessel[8] was critical of the EAA model and brought out that the EAA derived by conveniently using only a part of the Alveolar Gas Equation was not how it was originally meant to be used. Further, he argues that NH can never be considered identical to HH despite achieving an equivalent PiO2.
The CADO exposure did not require prebreathing of 100% oxygen for 30 min. Further, the time taken to reach 10,000 ft in the hypobaric chamber during CADO was shorter than the time required to reach 25,000 ft during HH. Thus, the CADO exposure used up considerably lesser time and oxygen compared to HH.
The findings of the present study demonstrated that CADO compared well with HH in inducing changes in physiological parameters and inducing psychomotor performance impairment. However, the symptoms experienced during HH were higher in incidence and severity compared to CADO. The higher incidence and severity of symptoms during HH could be attributed to the effects of hypobaria in itself, which has already been documented in the literature.
CONCLUSION
The appearance of symptoms and the subjects’ ability to remember and respond to them in the future is the principal basis of hypoxia awareness training. Therefore, CADO achieved by a combination of 10,000 ft and a hypoxic gas mixture of 10.3% oxygen and 89.7% nitrogen cannot be considered equivalent to HH as a tool for the hypoxia indoctrination of aircrew or CFF candidates. However, the combination mentioned above can be used for the demonstration of hypoxia to population subsets not on flying duties, as experiencing symptoms with diminished severity will not hamper or lessen the training objective as well as eliminate the risk of DCS during the CADO exposure. CADO can also be used effectively in simulating hypoxia in high-altitude research.
Future studies could look at the possibility of altering the combination of hypobaric and NH used to achieve CADO, by increasing the hypobaric component to a sufficient level; enough to increase the component of hypobaria while not crossing the threshold altitude for DCS.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Sponsored by Indian Society of Aerospace Medicine (ISAM).
References
- Hypoxia and hyperventilation In: Rainford DJ, ed. Ernsting's Aviation and Space Medicine (5th ed). Boca Raton, FL: CRC Press; 2016. p. :49-64.
- [CrossRef] [Google Scholar]
- Respiratory physiology and protection against hypoxia In: Davis JR, Johnson R, eds. Fundamentals of Aerospace Medicine (4th ed). Philadelphia, PA: Lippincott Williams and Wilkins; 2008. p. :20-45.
- [Google Scholar]
- Hypoxia symptoms in military aircrew: Long-term recall vs. acute experience in training. Aviat Space Environ Med. 2008;79:54-7.
- [CrossRef] [PubMed] [Google Scholar]
- Altitude Chamber Training: Is it Worth the Risk? Vol 39. United States: Flight Safety Foundation. Human Factors and Aviation Medicine; 1992.
- [Google Scholar]
- Hypoxia awareness training for aircrew: A comparison of two techniques. Aviat Space Environ Med. 2010;81:857-63.
- [CrossRef] [PubMed] [Google Scholar]
- The reduced oxygen breathing paradigm for hypoxia training: Physiological, cognitive, and subjective effects. Aviat Space Environ Med. 2001;72:539-45.
- [Google Scholar]
- Acute mountain sickness: Increased severity during simulated altitude compared with normobaric hypoxia. J Appl Physiol (1985). 1996;81:1908-10.
- [CrossRef] [PubMed] [Google Scholar]
- Critique of the equivalent air altitude model. Aviat Space Environ Med. 2008;79:975-82.
- [CrossRef] [PubMed] [Google Scholar]
- Normo-and hypobaric hypoxia: Are there any physiological differences? Eur J Appl Physiol. 2003;89:122-6.
- [CrossRef] [PubMed] [Google Scholar]
- A theoretical study of the composition of the alveolar air at altitude. Am J Physiol. 1946;146:637-53.
- [CrossRef] [PubMed] [Google Scholar]
- Alveolar gas composition at altitude In: A Graphical Analysis of the Respiratory Gas Exchange. Washington, DC: The American Physiological Society; 1955. p. :27-9.
- [Google Scholar]
- IAM Cognitive Test Battery: pSuMEDhA User Manual [User Manual] Bengaluru: IAM; 2018.
- [Google Scholar]
- The Cardiovascular system at high altitude In: Hornbein T, Schoene R, eds. High Altitude An Exploration of Human Adaptation. Boca Raton: CRC Press; 2001. p. :235-92.
- [Google Scholar]
- Hypoxic hypoxia and brain function in military aviation: Basic physiology and applied perspectives. Front Physiol. 2021;12:665821.
- [CrossRef] [PubMed] [Google Scholar]
- A closed-loop reduced oxygen breathing device for inducing hypoxia in humans. Aviat Space Environ Med. 2003;74:1190-7.
- [Google Scholar]
- Hypobaric and normobaric hypoxia training in aircrew: A comparative study. Ind J Aerospace Med. 2013;57:28-36.
- [Google Scholar]
- Cardiopulmonary response to acute altitude exposure: Water loading and denitrogenation. Respir Physiol. 1983;54:363-80.
- [CrossRef] [PubMed] [Google Scholar]
- Role of hypobaria in fluid balance response to hypoxia. High Alt Med Biol. 2005;6:60-71.
- [CrossRef] [PubMed] [Google Scholar]
- Ventilation, oxidative stress, and nitric oxide in hypobaric versus normobaric hypoxia. Med Sci Sports Exerc. 2013;45:253-60.
- [CrossRef] [PubMed] [Google Scholar]
- Ventilation during simulated altitude, normobaric hypoxia and normoxic hypobaria. Respir Physiol. 1997;107:231-9.
- [CrossRef] [PubMed] [Google Scholar]
- Hyperoxia and hypoxic hypoxia effects on simple and choice reaction times. Aerosp Med Hum Perform. 2017;88:1073-80.
- [CrossRef] [PubMed] [Google Scholar]
- Moderate intermittent hypoxia: Effect on two-choice reaction time followed by a significant delay in recovery In: Proceedings of the Human Factors and Ergonomics Society Annual Meeting. Vol 53. No. 20. Sage CA: Los Angeles, CA: SAGE Publications; 2009. p. :1564-8.
- [CrossRef] [Google Scholar]
- Effects of simulated high altitude on renin-aldosterone and Na homeostasis in normal man. J Appl Physiol. 1972;33:204-10.
- [CrossRef] [PubMed] [Google Scholar]
- Increase in blood-brain barrier permeability by altitude decompression. Aviat Space Environ Med. 1987;58:1082-6.
- [Google Scholar]






