Translate this page into:
Autonomic Modulation with Repeated Exposure to +Gz Acceleration
Abstract
Physiological (cardiovascular) adaptation to repeated Gz exposures has been demonstrated previously. However, the mechanism of this adaptation is still unclear. It is hypothesized that an improved autonomic function infrequently G exposed subjects is an underlying mechanism for this adaptation. The purpose of this study was to test this hypothesis that repeated exposure to +Gz acceleration is associated with improved autonomic responses during orthostatic stress. 16 male non-aircrew subjects underwent 70° head up tilt test on two separate occasions, before and after 3 exposures to gradual onset rate +Gz acceleration. Autonomic function during the head up tilt test was measured using Heart Rate Variability (HRV) frequency domain indices. The HRV indices were compared before and after three Gz exposure sessions given over 3 consecutive days. Gz tolerance levels and heart rate changes during +Gz exposures were analyzed. Compared with pre-exposure. G-exposed subjects demonstrated no change in HRV indices indicating no change in autonomic response or sympatho-vagal balance during orthostatic stress test. The +Gz tolerance levels over 3 consecutive +Gz exposures also did not change significantly. Heart rate changes during G showed no significant differences over different +Gz exposures. Physiological (Cardiovascular) adaptation to repeated Gz exposures could not be demonstrated in this study. Autonomic responses to orthostatic stress showed no significant change after 3 consecutive G exposures. Further experiments with maximal stimulation of autonomic function with increased duration and magnitude of Gz exposure is recommended.
Keywords
G exposures, Heart Rate Variability
Orthostatic stress
Introduction
Pilots of high performance aircrafts are routinely exposed to +Gz acceleration (G). Exposure to +GZ forces leads to activation of cardiovascular compensatory mechanisms [1]. Recent observations suggest that frequent G exposures are associated with physiological adaptation. This adaptation to repeated G exposures have been demonstrated in several ways. Newman et. al. [2,3] has suggested that 4-Gz-adapted fighter pilots exhibit an improved orthostatic response in terms of increase in magnitude of heart rate and blood pressure response to orthostatic stress. Convertino et al [4,5,6] demonstrated that repeated G exposures leads to an improved blood pressure regulation during cardiovascular stress and an improved G tolerance. A reduction in G tolerance following layoff from flying even for a week has also been described in high performance aircraft pilots. This is termed G-lay off [1,7]. It has been suggested that the physiological adaptation which would have taken place during routine G exposures is lost during lay-offperiod [7].
The physiological basis of this adaptation is still unclear. A standard text book of Aviation Medicine [1] states that “some” physiological adaptation occurs, which is inconclusive. Few authors have hypothesized that this adaptation is due to improved autonomic function upon frequent G exposures [2,4,5,6]. Recently, an increase in blood volume following frequent G exposures has also been suggested [8]. However, little experimental data exist to corroborate these observations. Therefore, this study was conducted with an aim to test the hypothesis whether repeated G exposure is associated with a change in autonomic function.
The autonomic function is a representation of the sympathetic and parasympathetic divisions of the autonomic nervous system (ANS). Amongst the various methods, heart rate variability (HRV) is a very useful tool for understanding the modulation of ANS [9,10] .It is a widely used method to evaluate sympathetic and vagal influences on the heart.
HRV can be measured by time and frequency domain analysis of heart rate (RR) intervals in the electrocardiogram (ECG).Frequency domain analysis measures separate rhythmic contributions from sympathetic and parasympathetic autonomic activity that modulate heart rate. Sympathetic activity is associated with the low frequency range (0.04-0.15 Hz) while parasympathetic activity is associated with the higher frequency range (0.15-0.4 Hz) of modulation frequencies of the heart rate. This difference in frequency ranges allows HRV analysis to separate sympathetic and parasympathetic contributory evidence [9,10].
Three main spectral components are distinguished in a HRV spectrum calculated from short recordings of 2 to 5 minutes. These are very low frequency (VLF) (upto 0.04 Hz), low frequency (LF) (0.04 - 0.15 Hz) and high frequency (HF) (0.15-0.40 Hz) components [task force]. The HF component reflecting momentary respiratory influences on the heart rate, is decreased by tilting or parasympathetic blocking drugs and is increased by sympathetic blocking drugs or controlled respiration. Therefore, the HF component in humans has been thought to provide a quantitative and specific index of vagal modulation [9,11]. On the other hand, the LF component is increased while standing and the increase is blocked by intravenous propranolol. Moreover, this component is not found in quadriplegic humans who have severe dysfunction of the sympathetic nervous system [11,12]. Therefore, the LF component has been interpreted as an indicator mainly of sympathetic influences (especially when expressed in normalised units) [9]. Interpretation of LF components is, however, controversial. It has been suggested by some to include both sympathetic and vagal influences [9].Consequently. the LF/HF ratio has been reported to be a convenient index of sympatho-vagal interaction [9,11]. The physiological explanation of VLF component is much less defined and the existence of a specific physiological process attributed to these heart rate changes might even be questioned [9]. The non-harmonic component which does not have coherent properties and is affected by algorithms of baseline or trend removal, is commonly accepted as a major constituent of VLF. Thus VLF assessed from short-term recordings is a dubious measure and is recommended to be avoided when HRV of short term ECG is interpreted [9.11].
Measurement of VLF, LF and HF power componentsis usually made in absolute values of power, but LF and HF may also be measured in normalized units (n.u.) [9] which represent the relative value of each power component in proportion to the total power minus the VLF component. The representation of LF and HF in n.u. emphasizes the controlled and balanced behavior of the two branches of the autonomic nervous system. Moreover, normalization tends to minimize the effect on the values of LF and HF components of the changes in total power.
Resting HRV has long been used to evaluate autonomic function. However, with so many external factors able to affect resting heart rate, HRV during orthostatic stress using head up tilt test provides a more effective and reliable method [11,13].HRV during head up lilt has been suggested as an effective method to evaluate autonomic function in pilots [14].In this study, the interaction of autonomic function components before and after G exposures was studied by HRV measures during a 70° head up tilt test.
Head up tilt (HUT) test has been considered the “gold standard” as an orthostatic stress test among clinical laboratory diagnostic studies in the evaluation of orthostatic intolerance [15]. A 70° head up tilt table test has been commonly used. Other tests for orthostatic tolerance include lower body negative pressure (LBNP) and passive standing test. It is found that heart rate responses during LBNP are subject to marked day to day variability [16].The cardiovascular effects of Gz stress are mainly due accentuation of hydrostatic pressure gradient from head to toe, where carotid and aortic baroreceptors are differentially stimulated [1,16]. LBNP causes stimulation of both carotid and aortic baroreceptors to an equal degree unlike head up tilt test, where carotid baroreceptors are stimulated predominantly [1,17]. The order of stimulation of baroreceptors during LBNP therefore differs from that of head up tilt and Gz stress [17]. Thus, head up tilt test is a better test to simulate G stress. On the other hand, although a passive standing test is simple and does not require any apparatus, it has been generally objected to. This is because of the likelihood that apposition of the feet to a firm surface would allow activation of the muscle-vein pump in the lower extremities. This can increase venous return and thereby improve orthostatic tolerance [16]. Hence, head up tilt test was chosen as an orthostatic stress test for evaluation of autonomic responses following Gz exposures.
Materials and method
Subjects
Sixteen non-aircrew male subjects with mean age 29.5 ±3.3 years, weight of 76.3 ± 7.55 kg and height of 173.4 ± 6.6 cm volunteered to participate as subjects for tills experiment after all procedures and risks associated were explained. Their voluntary written informed consent to participate in the study was obtained. The study design and the protocol for the study was approved by the Institute Ethics Committee. After a detailed history, a thorough clinical evaluation of the subjects was done before they participated in the experiment. Subjects were asked to refrain from exercise and stimulants such as caffeine and other drugs six hours before testing. During an orientation period that preceded the experiments, subjects were made familiar with the laboratory, the protocol and procedures. They had the option of withdrawing from the study at any stage during the experiment.
Inclusion criteria:
Healthy male individuals
Normotensive
No prior history of G exposure
Age between 20 to 40 years
Exclusion criteria:
History of any disease, infirmity or severe motion sickness
Aerobically trained individuals
Aircrew
Experiment Protocol: The experiment was conducted over three days and consisted of two head up tilt tests which were done before and after G exposures over three consecutive days.
Day I: Head Up Tilt test followed by first G exposurein the Human Centrifuge
Day 2: Second G exposure in the Human Centrifuge.
Day 3: Third G exposure in the centrifuge followed by 70° Head Up Tilt test. The orthostatic stress test for each subject was done within 24h of the beginning and the end of three +Gz exposures.
Material and Methods
Head Up Tilt Test:
The Huntleigh AKRON 9622 multipurpose tilt table with footboard support in the Department of Space and Environmental Physiology at Institute of Aerospace Medicine, Bangalore was used for orthostatic stress test. Following a restful night, the test was performed between 10 am and 11 am, to avoid circadian influences on the response of the cardiovascular system to HUTT. All subjects had abstained from alcohol throughout the study and from smoking on the morning of the test. The tilt was administered at least 2 hr after breakfast. The room temperature was maintained between 23-26° Celsius. The room was dimly illuminated. Each subject was strapped supine onto the tilt table andrestraint system was provided for subject safety. Three chest electrodes for ECG recording were attached, one under left clavicle, one under right clavicle and another just below the apex of the heart. The leads from Nexus-10 were connected to the electrodes. The instrument transferred data to a computer via Bluetooth. The monitoring screen displayed continuous ECG and beat to beat heart rate changes. The sampling of ECG was set at 1024 samples per second. Continuous beat to beat heart rate changes and electrocardiogram was recorded using Nexus-10 instrument. On reaching a steady state (5 min), the subject was rapidly tilted to 70° head-up position in 10-12 seconds. The subject was tilted back to supine after 5 minutes at 70°. ECG recording continued for 5 min in the post-HUT period in supine posture. Marking of each event during tilting was also done.
Simulation of Gz acceleration
+Gz acceleration was simulated in the High Performance Human Centrifuge in the Dept of Acceleration Physiology and Spatial Orientation at the Institute of Aerospace Medicine. The centrifuge was configured with an upright seat that was adjusted to 13° seat-back position with a 54-56° light bar. The light bar consisted of a central red light and peripheral green lights. The subjects were exposed to gradual onset rate (GOR) +Gz acceleration at an onset rate of 0.1 G per second. Subjects did not wear any anti-G suit during +Gz exposures. Continuous ECG recoding was done throughout the centrifuge runs. During the centrifuge run. the subject remained relaxed till the peripheral green lights disappeared (Peripheral Light Loss) which was indicated by the subject by releasing the dead man switch (DMS) provided at the central stick. The profile was terminated any time if the subject felt any discomfort, nausea, vomiting, neck or back pain, or in case of an inadvertent or impending loss of consciousness.
The following criteria were established for stopping a run earlier than scheduled time:
Subject’s choice: viz, at his own discretion, the subject could stop the run by releasing the DMS.
Medical: viz, if the medical officer monitoring the run observed gross abnormalities in the ECG.
Technical: viz, if a failure occurred in the data system, so that the medical monitor was unable to guarantee the subject’s safety.
Previous studies evaluating effects of repeated Gz exposures have exposed aircrew and non aircrew subjects to Gz ranging from 3 days to 3 weeks. However, die number of G exposures and the number of days they are required for physiological adaptation to occur are not specified in literature. In this study. +Gz exposures were limited to three which were given over three consecutive days, considering operational constraints for the centrifuge usage.
Autonomic function
Autonomic function was measured using Heart Rate Variability. ECG recorded with a sampling rate of 1024 Hz during the head up tilt test was selected for HRV analysis. Short term recording of frequency domain indices require at least 5 minute epoch (task force. 5 min duration), which is widely used in research. ECG recording of 5 minutes during the pre-tilt supine, 70° tilt and post-tilt supine each were selected for HRV analysis using Nexus 10 Biotrace + software. Inter beat intervals (IBI) were obtained after artifact correction. The inter beat intervals were further analysed using the Kubios HRV, Heart Rate Variability (HRV) analysis software. The RR interval time series was interpolated at 4Hz to produce a continuous time signal for power spectrum analysis. The spectral components of the data were obtained by Auto-regression analysis. The high frequency (HF) power spectrum was evaluated in the range from 0.15 to 0.4 Hz. The low frequency (LF) power spectrum was evaluated in the range from 0.04 to 0.15 Hz. Their power was studied after subtraction of the VLF range and normalisation.
Normalized power (n.u.) is power of LF and HF bands in normalized units. This is derived by;
LF (n.u.) = LF (ms2) / [total power (ms2) VLF (ms2)]
HF (n.u.) = HF (ms2)/[(total power (ms2) VLF (ms2)]
Only normalized HRV indices are presented in the results
Statistical analysis: Paired ‘t’ test was used to compare HRV indices before and after G exposures and delta heart rate during G exposures. Repeated measure ANOVA with post hoc analysis using Tukey’s multiple comparison was used to analyze G tolerance over 3 days and heart rate at different G levels. The significance level was set at p<0.05.
Results
HR V during head up tilt test
The frequency domain indices of HRV during different stages of head up tilt test before and after Gz exposures are presented in Table 1. The change in posture from supine to 70° tilt reproduced an increase in sympathetic activity shown by an increase in nLF component of HRV and withdrawal of parasympathetic activity by a decrease in nHF component. nLF%, nHF% and LF/HF ratio during 5 minutes of supine, 70° tilt and post-tilt supine period were compared before and after Gz exposures. There were no significant differences during any period of tilt test as shown in Table 1.
| Pre-G exposures | Post-G exposures | P value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HRVIndices | Pre-tilt | Tilt | Post-tilt | Pre-tilt | Tilt | Post-tilt | Pre-tilt | Tilt | Post-tilt |
| nLF%(n.u.) | 74.4±10.4 | 81.4±9.5 | 67.9±12.1 | 69.9±10.9 | 81.45±1.7 | 63.55±11.5 | 0.055(ns) | 0.993(ns) | 0.2649(ns) |
| nHF%(n.u.) | 25.5±1 | 18.5±2.3 | 34±12 | 29.6±10.6 | 19±1.6 | 36.9±11.8 | 0.071(ns) | 0.849(ns) | 0.510(ns) |
| LF/HF | 3.65±0.7 | 6.12±1.1 | 2.83±0.2 | 3.11±0.3 | 5.2±0.5 | 2.03±0.1 | 0.171(ns) | 0.446(ns) | 0.129(ns) |
Gz exposures
The relaxed Gz tolerance levels of three G exposures over three consecutive days are presented in Table 2. The G tolerance level remained unchanged over the three days (Table 3).
| Day 1 | Day 2 | Day 3 | |
|---|---|---|---|
| Mean ± SD | 4.34(±0.76) | 4.10(±0.67) | 4.16(±0.67) |
| Range | 2.9-5.6 | 2.9-5.2 | 3.0-5.4 |
| One-way analysis of variance Relaxed Gz tolerance | ||||
|---|---|---|---|---|
| P value | 0.1943 | |||
| F | 1.731 | |||
| R square | 0.1035 | |||
| Post hoc analysis Relaxed Gz tolerance | ||||
| Tukey’s Multiple Comparison Test | Mean Diff. | q | Significant? p < 0.05? | 95%CIofdiff |
| DAY 1 RLX vs DAY 2 RLX | 0.2375 | 2538 | No | -0.08865 to 0.5637 |
| DAY 1 RLX vs DAY 3 RLX | 0.1750 | 1.870 | No | -0.1511 to 0.5012 |
| DAY 2 RLX vs DAY 3 RLX | -0.0625 | 0.6680 | No | -0.3887 to 0.2637 |
Hear rate changes during G
Heart rate at different G levels over three Gz exposures were analyzed. Heart rate at 1G, 2G and 3G were compared over three days of Gz exposure (Table 4.5.6). There were no significant differences in the heart rate values at respective G levels.
| One-way analysis of variance of Heart rate at 1 G | ||||
|---|---|---|---|---|
| p value | 0.5825 | |||
| F | 0.5511 | |||
| R square | 0.03787 | |||
| Post hoc analysis of Heart rate at 1 G | ||||
| Tukey’s Multiple Comparison Test | Mean Diff. | q | Significant? P<0.05? | 95% CI of diff |
| Day 1 vs Day 2 | 0.6667 | 0.3299 | No | -6.409 to 7.742 |
| Day 1 vs Day 3 | -2.200 | 1.089 | No | -9.276 to 4.876 |
| Day 2 vs Day 3 | -2.867 | 1.419 | No | -9.942 to 4.209 |
| One-way analysis of variance of Heart rate at 1 G | ||||
|---|---|---|---|---|
| p value | 0.8242 | |||
| F | 0.1946 | |||
| R square | 0.01371 | |||
| Post hoc analysis of Heart rate at 1G | ||||
| Tukey’s Multiple Comparison Test | Mean Diff. | q | Significant? P<:0.05? | 95% CI of diff |
| Day 1 vs Day 2 | -2.133 | 0.8818 | No | -10.60 to 6.338 |
| Day 1 vs Day 3 | -1.133 | 0.4684 | No | -9.605 to 7.338 |
| Day 2 vs Day 3 | 1.000 | 0.4133 | No | -7.471 to 9.471 |
| One-way analysis of variance of Heart rate at 1 G | ||||
|---|---|---|---|---|
| p value | 0.3245 | |||
| F | 1.172 | |||
| R square | 0.07724 | |||
| Post hoc analysis of Heart rate at 1 G | ||||
| Tukey’s Multiple Comparison Test | Mean Diff. | q | Significant? p < 0.05? | 95% CI of diff |
| Day 1 vs Day 2 | 0.9333 | 0.3244 | No | -9.140 to 11.01 |
| Day 1 vs Day 3 | -4.867 | 1.692 | No | -14.94 to 5.207 |
| Day 2 vs Day 3 | -5.800 | 2.016 | No | -15.87 to 4.273 |
A difference in heart rate at G tolerance level and 1G was measured (Ä HR). There was no significant difference in Ä HR over the three Gz exposures (Table 7).
| Day 1 (bpm) | Day 3 (bpm) | test of significance (p value) | |
|---|---|---|---|
| Ä HR | 48±19 | 52±15 | 0.231 (ns) |
Correlation of Ä HR with Gz tolerance on day 1 showed no significant correlation (Fig 1) (r= -0.15, p=0.56). On day 3 the correlation showed minimal change to that on day 1 (r = -0.29. p=0.27) (Fig 2).

- Correlation of +Gz tolerance with change in heart rate from 1G to G tolerance - Day 1 (n= 16)
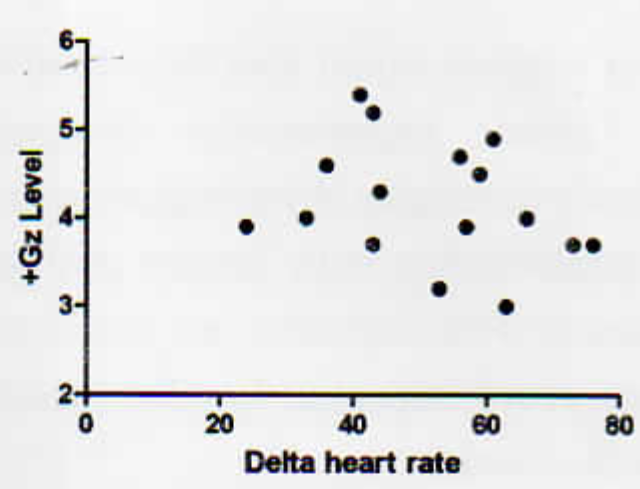
- Correlation of +Gz tolerance with change in heart rate from 1G to G tolerance - Day 3 (n= 16)
Discussion
The present study was conducted to test the hypothesis that repeated exposure to Gz acceleration would be associated with differences in autonomic functions. The major findings in this study do not support this hypothesis.
Following three sessions of G exposure over 3 consecutive days, the sympathetic and parasympathetic response to orthostatic stress test showed no significant changes. This has been shown by the frequency domain indices of HRV during different stages of the head up tilt test (Table 1). The lack of change in autonomic response following G exposures would suggest either that a physiological adaptation (which has been suggested by several authors) has not occurred or the mechanism of adaptation was not evident through a change in autonomic function.
In addition, Gz tolerance levels did not change significantly over the three G exposures (Table 2,3). Other studies investigating effect of repeated G exposure on G tolerance have demonstrated an increase in +GZ tolerance [4,5,6]. It is reasonable to suggest that the unchanged G tolerance level over 3 exposures indicate lack of physiological adaptation to G.
It is of interest that some underlying mechanism of cardiovascular adaptation can also be deduced from heart rate changes during G exposures. Heart rate increases in response to G. It has been suggested that at low G levels the heart rate is directly correlated with the level and duration of G exposure. In the present study, the heart rate at different G levels was compared over three G exposures. This, however, showed no significant difference (Table 4,5,6). This suggests a lack of change in autonomic response to Gz stress over 3 days of G exposures.
The difference in heart rate (Ä HR) from 1 G to G tolerance level can be considered as a measure of autonomic function. The Ä HR did not change significantly over 3 G exposures (Table 7). Also, the correlation of Ä HR with G tolerance showed no significant difference on day 1 and day 3 of G exposure (Fig 1 ,2). On the other hand, it can be hypothesized that the G tolerance level is independent of increase in heart rate from baseline to G tolerance level (Ä HR).
Together, the data provides indirect evidence that physiological adaptation to three Gz exposures has not occurred. However, the notion that adaptation following repeated G exposures occurs cannot be entirely ruled out. It could be probable that the magnitude and duration of G exposures were not sufficient enough to induce cardiovascular adaptations. Although a previous study has demonstrated cardiovascular adaptation after 3 days of G exposure [5], the magnitude of G exposed was higher. The subject strength for the study could be a limiting factor with less than optimal number of subjects.
Conclusion
This study was an attempt to evaluate autonomic function after repeated +Gz exposures and to test the hypothesis that repeated G exposures would enhance orthostatic stress response. This hypothesis was not supported in the present study. It is evident from the study that a 3 day exposure to gradual onset rate +Gz acceleration neither did bring about a change in autonomic response to orthostatic challenge as measured by HRV, nor a change in +Gz tolerance. The heart rate response to repeated +Gz stress and a change in response also remained unaltered. Although not evident from the present study, physiological adaptation to chronic +Gz acceleration cannot be entirely ruled out. Absence of change in autonomic function in this present study could be due to lack of maximal stimulation of autonomic system, which can be suggested to occur with exposure to high G and a longer duration of exposure to +Gz stress. Less than optimum number of subjects could also be a contributing factor. Further experiments designed to systematically investigate physiological mechanisms of this relationship with longer and greater magnitudes of +Gz exposures are recommended. This could prove important in pilot training and combat effectiveness.
References
- Effects of long duration acceleration In: Rainford DJ, Gradwell DP, eds. Ernsting’s Aviation Medicine (4th ed). New York: Edward Arnold Ltd; 2006. p. :137-58.
- [Google Scholar]
- Evidence of baroreflex adaptation to repetitive +Gz in fighter pilots. Aviat Space Environ med. 1998;69:446-51.
- [Google Scholar]
- The effect of Baroreflex Adaptation on the Dynamic Cardiovascular Response to Head-Up Tilt. Aviat Space Environ Med. 2000;71:255-9.
- [Google Scholar]
- High sustained +Gz acceleration: Physiological adaptation to high-G tolerance. J Gravit Physiol. 1998;5:51-4.
- [Google Scholar]
- Mechanisms of blood pressure regulation that differ in men repeatedly exposed to high-G acceleration. Am J Physiol Regulatory Integrative Comp Physiol. 2001;280:947-958.
- [Google Scholar]
- Gender differences in autonomic functions associated with blood pressure regulation. Am J Physiol Regulatory Integrative Comp Physiol. 1998;275:1909-20.
- [Google Scholar]
- Effects of G-layoff on subsequent tolerance to +Gz [Abstract] Aviat Space Environ Med. 1994;65:448.
- [Google Scholar]
- Cardiovascular training effects in fighter pilots induced by occupational high G exposure. Aviat Space Environ Med. 2008;79:774-8.
- [Google Scholar]
- Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability Standards of measurement, physiological interpretation, and clinical use. European Heart Journal. 1996;17:354-381.
- [Google Scholar]
- Respiration and Heart rate variability: A review with special reference to its application in Aerospace Medicine. Ind J Aerospace Med. 2004;48(1):64-75.
- [Google Scholar]
- Power spectral analysis of heart rate variability in traumatic quadriplegic. Am J Physiol Heart CircPhysiol. 1990;258(H1):722-6.
- [Google Scholar]
- Power spectrum analysis of heart rate variability to assess the changes in sympathovagal balance during graded orthostatic tilt. Circulation. 1994;90:1826-1831.
- [Google Scholar]
- Evaluation of the effect of flights in supersonic fighters on sinus rhythm variability parameters. Mil Med. 2003;168(2):91-5.
- [Google Scholar]
- Tilt table testing for assessing syncope. American College of Cardiology. J Am CollCardiol. 1996;28:263-275.
- [Google Scholar]
- Comparison of 70° tilt LBNP and passive standing as measures of orthostatic tolerance. Aviat Space Environ Med. 1975;46:801-808.
- [Google Scholar]
- The physiology of positive acceleration In: Gillies JA, ed. A text book of Aviation Physiology. Oxford: Pergamon press; 1965. p. :551-687.
- [Google Scholar]






