Translate this page into:
Simultaneous analysis of ten drugs of abuse in blood and urine matrix by gas chromatography–mass spectrometry: Implications for air crash investigation

*Corresponding author: Dr. SR Santhosh, Scientist ‘E’, HoD, Department of Aviation Pathology and Toxicology, Institute of Aerospace Medicine, IAF, HAL Old Airport Rd., Vimanapura, Bengaluru - 560 017, Karnataka, India. santhuvet4u@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Santhosh SR, Sampath S, Gupta A, Kumar A. Simultaneous analysis of ten drugs of abuse in blood and urine matrix by gas chromatography–mass spectrometry: Implications for air crash investigation. Indian J Aerosp Med 2019;63(2):65-70.
Abstract
Introduction:
Many of abuse drugs can alter a person’s thinking and judgment, leading to health risks, including addiction, drugged driving and infectious disease. Use of illicit drugs by aviation employees is associated with a significantly increased risk of accidents. The detection and quantitation of drugs of abuse in blood is of growing interest in forensic and clinical toxicology. Generally, the screening for drugs of abuse is carried out by using commercially available immunoassay based urine cassettes; however, such results needs to be confirmed by advanced analytical tools like HPLC, GCMS and LCMS. There have been several attempts to develop confirmatory methods for drugs of abuse in blood and urine.
Material and Methods:
In the present study in our laboratory, a single method was attempted for simultaneous detection and quantification of 10 drugs of abuse in whole blood and urine matrix by GC-MS Selective Ion Monitoring (SIM) method.
Results:
Chromatographic separation was optimized and achieved for separation of all 10 compounds using Agilent DB-5MS column. Retention time (Rt), selectivity and sensitivity were achieved by measuring each analyte in Selective Ion Monitoring (SIM) mode. A simple sample preparation method was standardized for extraction of all 10 compounds from blood and urine.
Conclusion:
The developed method in the study permits identification of these analytes from same biological specimen in small quantities and the method is tested on blood and urine matrix spiked with known concentration of pure compounds.
Keywords
Drugs of abuse
GCMS
Aircraft accident investigation
INTRODUCTION
When controlled substances are used in a manner or amount inconsistent with legitimate medical use, it is called drug abuse. The non-sanctioned use of substances controlled under Schedule I drugs is considered drug abuse. In addition to having abuse potential, most controlled substances are capable of producing dependence, either physical or psychological. The list of “drugs of abuse” can vary depending on who is performing the analysis, i.e., clinical toxicology, forensic toxicology, workplace testing, doping analysis in humans and animals, or rehabilitation programs focus on different “drugs of abuse.” The determination of the most commonly occurring illicit drugs and their metabolites which are important for the assessment of drug abuse are methamphetamine, amphetamine, 3,4-methylenedioxymethamphetamine, (MDMA), N-ethyl-3,4-methylenedioxyamphetamine (MDEA), 3,4-methylenedioxy-amphetamine, (MDA), cannabinoids (delta-9-tetrahydrocannabinol, 11-hydroxy-delta-9-tetrahydrocannabinol, 11-nor-9-carboxydelta-9-tetrahydrocannabinol), cocaine, benzoylecgonine, ecgonine methyl ester, cocaethylene, and the opiates (Heroin,6-monoacetylmorphine, morphine, codeine, and dihydrocodeine).[1]
The use of illicit drugs by aviation employees is associated with a significantly increased risk of accident involvement. Illicit drugs – such as amphetamine, cannabis, cocaine, and methamphetamine – detected in the deceased pilots could be linked to their unauthorized use by them.[2] Therefore, developing an effective method for the simultaneous analysis of these drugs (and their metabolites) is an inescapable need as a part of a toxicological investigation of post-crash events.
Typically, the blood and urine are the most commonly used biological specimen for analysis of drugs of abuse in forensic science and clinical chemistry although there are several other specimens which are less commonly used. Gas chromatography-mass spectrometry (GC-MS) methodology has long been used for the analysis of opiates,[3] methamphetamine,[4,5] ketamine,[6,7] and related drugs. However, simultaneous analysis of these drugs and their metabolites by GC-MS is challenging because it is difficult to develop a “single extraction and chemical derivatization protocol” that could work optimally for all analytes.[8,9] Amphetamines and opiates are known to undergo metabolism in body and many times instead of the parent compounds its metabolites are excreted in the urine.
An aircraft accident investigation is the unique and important role of the Department of Aviation Pathology and Toxicology at the Institute of Aerospace Medicine as this department is a nodal center in India for conducting air crash investigation of all fatal air crashes involving both Civil and Military aircraft. Post-crash samples are usually whole blood and urine. This study was undertaken with the broad objectives of developing a GC-MS-based detection tool for estimation of commonly used drugs of abuse in whole blood and urine so that it can be employed in toxicological screening of autopsy specimens of fatal air crashes.
MATERIAL AND METHODS
Opiate multi-component mixture-5 (codeine, hydrocodeine, oxycodone, mepheridine, and methadone) solution containing 250 µg/mL of each component in ampule of 1 mL methanol from Cerilliant Sigma (#O-020), Methamphetamine (1 mg/mL) in methanol from Cerilliant Sigma (#M-009), MDA (1 mg/L) in methanol from Cerilliant Sigma (#M-012), MDMA (1 mg/L) in methanol from Cerilliant Sigma (#M-013), MDEA (1 mg/L) in methanol from Cerilliant Sigma (#M-065), and Trazodone (1 mg/L) in methanol from Cerilliant Sigma (#T-030) is used as certified reference material grade in the present study. These standards in the mixture were used to develop a single GC-MS SIM method for simultaneous detection and quantification of ten-drugs of abuse in spiked blood/urine matrix using the Agilent 7890A GC and 5975 C mass selective detector. Similarly, all solvents used were standardized for liquid chromatography– mass spectrometry (LC–MS) grade and reagents used were standardized for the American Chemical Society grade.
The standard operating procedures were used to develop a GC-MS method for benzodiazepines. Determination of retention time (Rt) and identification of candidate SIM ions, for all the compounds were determined as the first and utmost important steps in the method development. 100 ng of multi-component drug standard was injected and scanned from 100 to 600 amu on DB-5MS column for checking the spectrum of the compounds planned to analyze by SIM. All the peaks were characterized, Rt for each compound/peak was noted. Two ions (Target and Q1) for each compound were determined by analyzing the standard in the full scan mode following the procedure instructed by the instrument manufacturer. Ions (Target and Q1) for each compound were selected by examining the spectrum for candidate SIM ions. The ions that are unique to the compound, higher in mass and abundant in quantity were selected and tabulated.
The details of the scan mode method used to set up a SIM method for drugs of abuse. GC-MS conditions standardized for the GC-MS SIM method are as below:
| GC | Agilent 7890A with auto-injector and tray |
|---|---|
| Inlet | EPC split/splitless |
| Mode | Constant pressure |
| Injection type | Split |
| Split ratio | 5:1 |
| Split flow | 13.7 mL/min |
| Injection volume | 1.0 µL |
| Inlet temperature | 280°C |
| Septum purge flow | 3 mL/min |
| Gas type | Helium |
| Oven | |
| Equilibration time | 0.5 min |
| Initial oven hold | 100°C for 0.25 min |
| Ramp rate | 40°C/min |
| Final temp | 325°C |
| Run time | 9 min |
| Column | DB-5MS |
| MSD type | Agilent 5975 |
| Mode | SIM |
| Solvent delay | 0.7 min |
| Quad temperature | 180°C |
| Source temperature | 280°C |
| Transfer line temperature | 300°C |
SIM: Selective ion monitoring, GC: Gas chromatography, EPC: Electronic pneumatics control, MSD: Mass selective detector
After categorizing the method for separating all ten compounds on the Agilent DB-5MS column, a method to extract these drugs from whole blood and urine was developed. A series of calibration solutions were prepared in blood/urine of healthy persons with no previous history of consumption of medication by spiking “drugs of abuse standard mixture solution” having ten compounds. Briefly, 500 µL of human blood/urine was spiked with different volumes of “standard mixture stock solution” to prepare 250 ppb, 500 ppb, and 750 ppb final concentration of the drug mix in blood/urine matrix in a microfuge tube. To prepare a blank, 500 µL of blood/urine from a volunteer with no history of medication was used.
Bond Elut Captiva ND Lipid from Agilent (P/N: A5300635) along with Vac Elut 12 Manifold from Agilent (P/N: 5982– 9110) was used for solid-phase extraction. All the matrix-match-standards and blank were processed by a simple protein precipitation method adopted in the laboratory. In brief, 1500 µl of acetonitrile was added to 500 µl of blood or urine which spiked with a known concentration of standard drug mix and vertexed for 30 s. The supernatant was transferred to Bond Elut Captiva ND Lipid cartridges after centrifuge at 6000 rpm for 5 min at 8°C. The vacuum was applied and the elute collected was vacuum concentrated by nitrogen concentrator. The vacuum concentrated samples were reconstituted with 150 µl of toluene and injected into the system.
RESULTS
Chromatographic separation was optimized and achieved for all the ten target compounds using the Agilent DB-5MS column. Retention time (Rt), selectivity, and sensitivity were achieved by measuring each analyte in selective ion monitoring (SIM) mode [Table 1]. A simple protein precipitation method was used to extract target compounds from blood/urine samples. This method was quick and amenable to clinical research. Optimal separation of the selected compounds was achieved within a run time of <8 min [Figure 1]. All calibration curves were generated with linear curve fitting and were weighed (1/x). A linear dynamic range of 250–750 ng/mL was achieved for all the analytes with an R2 value >0.9 [Figure 2].
| Compounds | Rt (Min) | Detection window (Min) | Target ion (mass) | Q ion (mass) |
|---|---|---|---|---|
| Methamphetamine | 1.16 | 1.01–1.31 | 91 | 58 |
| MDA | 2.09 | 1.91–2.24 | 135 | 136 |
| MDMA | 2.26 | 2.11–2.41 | 135 | 58 |
| MDEA | 2.41 | 2.26–2.56 | 135 | 72 |
| Mepheridine | 2.96 | 2.81–3.11 | 246 | 172 |
| Methadone | 4.05 | 3.90–4.20 | 165 | 72 |
| Codeine | 4.65 | 4.30–4.80 | 162 | 299 |
| Hydro codeine | 4.81 | 4.66–4.96 | 242 | 299 |
| Oxycodone | 4.99 | 4.84–5.14 | 230 | 315 |
| Trazodone | 6.70 | 6.55–6.85 | 176 | 209 |
MDA: 3,4-methylenedioxy-amphetamine, MDMA: 3,4-methylenedioxymethamphetamine, MDEA: N-ethyl-3,4-methylenedioxyamphetamine, SIM: Selective ion monitoring, Rt: Retention time, GC-MSD: Gas chromatography–Mass selective detector

- Total ion chromatogram showing the separation of all ten drugs of abuse.
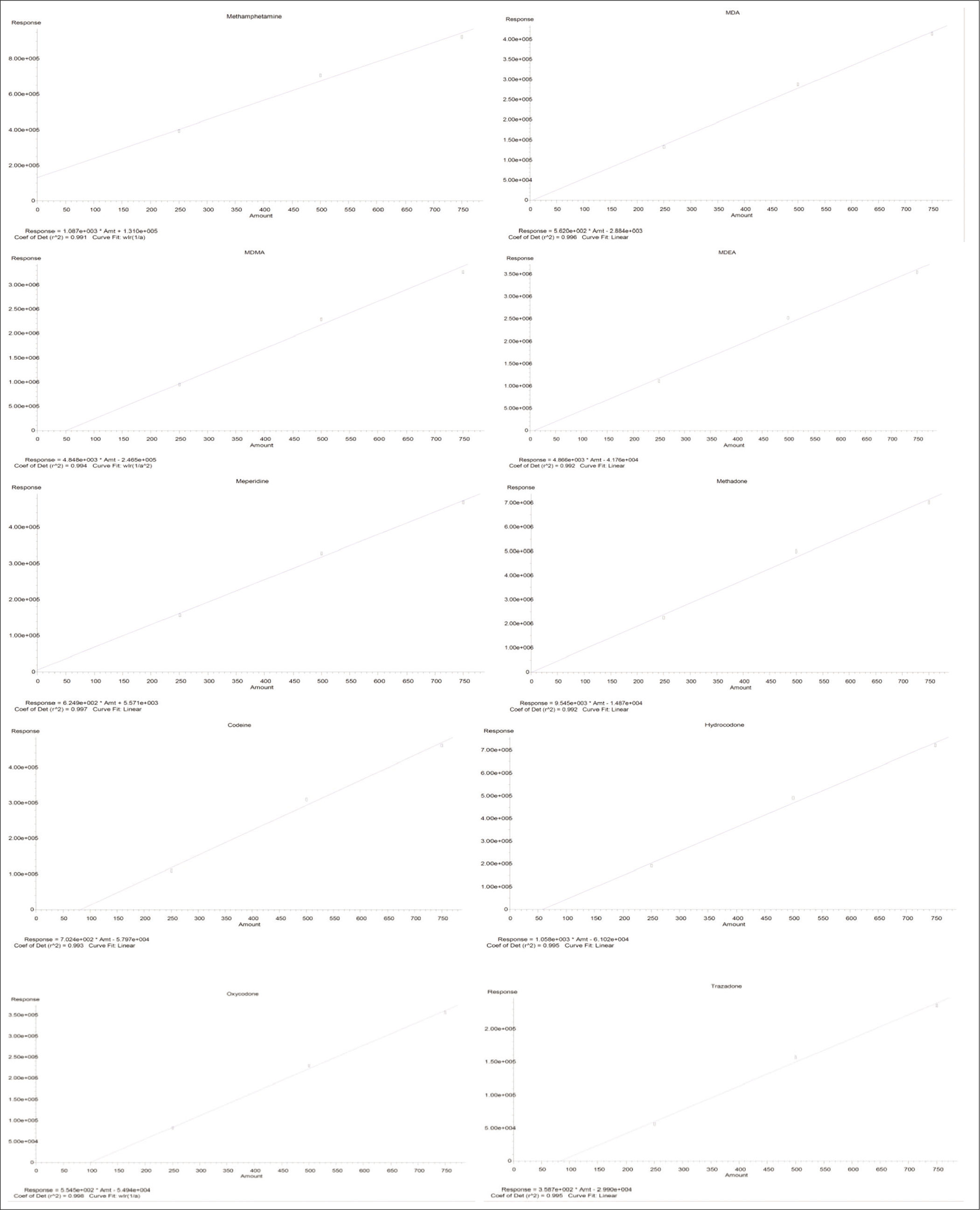
- Standard curves generated for all the ten drugs of abuse.
The standard curve thus obtained revealed a working range of 250–750 ppb. The CV was >0.9 with the sensitivity of 250 ppb for all compounds. Recovery study revealed a recovery of 80– 90% for all drugs. Repeatability study also revealed similar Rt, peak shape, and response after six injections. The specificity of the developed method was attributed to the Rt, product ions, and ion ratio.
DISCUSSION
Abuse of drugs is associated with numerous medical, social, and legal problems. “Drugs of abuse” testing is, therefore, an important task in forensic toxicology and related fields. It is generally performed either to confirm an acute drug effect (e.g., in drugged driving) or to monitor drug abstinence (e.g., in workplace drug testing).[10]
Civil Aerospace Medical Institutes, Federal Aviation Administration, USA studied the trends in illicit and prescription drug use in pilots of civil aviation accidents. The results are comparable to the general population seen in emergency departments and community data from major metropolitan areas. Of the 5321 pilots involved in aviation accidents during the examined time period, there were 467 occurrences of either illicit drugs or commonly abused prescription drugs accounting for 11% of all pilots that were involved in aviation accidents.[11]
The analytical methods used for confirmation tests are generally based on GC or LC coupled to single-stage or tandem mass spectrometry. The screening methods have a high incidence of false-positive and false-negative reports. Therefore, confirmation of screening techniques is required to assist in developing a definitive clinical diagnosis.
A number of confirmatory techniques can be used for the analysis of drugs of abuse; GC-MS is considered the gold standard technique for the identification of drugs of abuse in crime laboratories. GC-MS is a very sensitive analytical tool and has the advantage of relatively low-cost instrument and availability of drug identification libraries.[12]
Several laboratories have developed confirmatory methods for the determination of drugs of abuse from different biological samples by adopting different advanced analytical tools. Some of the laboratories developed a method for quantification of drugs of abuse in whole blood by GCMS and other laboratories have developed methods using high-performance-LC and LC-MS platforms also used with sensitivity ranging from 50 ng/mL to 250 ng/mL.[1,13-15]
The present study developed a method for the determination of ten drugs of abuse in blood and urine matrix using GCMS with the sensitivity of 250 ng/mL. The sensitivity of the developed method is slightly less compared to some of the previously developed methods. The present method used simple sample preparation without derivatization of the analytes. The previously published methods adopted a separate derivatization process to enhance sensitivity. However, when the method was validated on blood and urine samples spiked with the known concentration of drug mix, we could estimate all ten drugs with a recovery of around 80% and sensitivity of 250 ng/mL. Validation of the method on actual biological samples could not be done due to the paucity of specimens with a known history of consumption of the above drugs. The method developed in the present study can be further improved by adopting the derivatization process to enhance the sensitivity and by analyzing actual biological samples from the persons with a known history of consumption.
CONCLUSION
In the present study, a single method was developed for detection and quantification of ten-drugs of abuse in whole blood and urine matrix by GC-MS SIM method. The developed method adopted a simple sample preparation step without sample derivatization. Chromatographic separation was optimized and achieved for all the ten target compounds using the Agilent DB-5MS column. The developed method permits identification of multiple molecules or analytes from same biological specimen in small quantities. The practical use of this method is huge. Besides corroborating suspicion, it could also quantify the drug level and have several applications across several industries and situations. However, before the clinical application, the developed method needs to be validated on actual biological specimens.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Determination of drugs of abuse in blood. J Chromatogr B Biomed Sci Appl. 1998;713:91-109.
- [CrossRef] [Google Scholar]
- Drug violations and aviation accidents: Findings from the US mandatory drug testing programs. Addiction. 2011;106:1287-92.
- [CrossRef] [PubMed] [Google Scholar]
- GC-MS quantitation of codeine, morphine, 6-acetylmorphine, hydrocodone, hydromorphone, oxycodone, and oxymorphone in blood. J Anal Toxicol. 2005;29:301-8.
- [CrossRef] [PubMed] [Google Scholar]
- Simultaneous determination of methamphetamine and amphetamine in human urine using pipette tip solid-phase extraction and gas chromatography-mass spectrometry. J Pharm Biomed Anal. 2007;44:602-7.
- [CrossRef] [PubMed] [Google Scholar]
- Screening for and validated quantification of amphetamines and of amphetamine-and piperazine-derived designer drugs in human blood plasma by gas chromatography/mass spectrometry. J Mass Spectrom. 2003;38:659-76.
- [CrossRef] [PubMed] [Google Scholar]
- Gas chromatography-isotope dilution mass spectrometry preceded by liquid-liquid extraction and chemical derivatization for the determination of ketamine and norketamine in urine. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;799:37-50.
- [CrossRef] [PubMed] [Google Scholar]
- Simultaneous quantification of amphetamines, caffeine and ketamine in urine by hollow fiber liquid phase microextraction combined with gas chromatography-flame ionization detector. Talanta. 2010;82:969-75.
- [CrossRef] [PubMed] [Google Scholar]
- Gas chromatography-mass spectrometry analysis of ketamine and its metabolites-a comparative study on the utilization of different derivatization groups. J Chromatogr A. 2007;1157:336-51.
- [CrossRef] [PubMed] [Google Scholar]
- Enantiomeric separation and quantitation of (+/-)-amphetamine, (+/-)-methamphetamine, (+/-)-MDA, (+/-)-MDMA, and (+/-)-MDEA in urine specimens by GC-EIMS after derivatization with (R)-(-)-or (S)-(+)-alpha-methoxy-alpha-(trifluoromethy)phenylacetyl chloride (MTPA) J Anal Toxicol. 2004;28:449-55.
- [CrossRef] [PubMed] [Google Scholar]
- Controlled substance act: Controlling drugs or other substances through formal scheduling In: Drugs of Abuse-A DEA Resource Guide. U.S: US Department of Justice (Drug Enforcement Administration); 2017. p. :8-15.
- [Google Scholar]
- Drug usage in Pilots Involved in Aviation Accidents Compared with Drug Usage in the General Population: From 1990 to 2005 In: Final Report. Washington, DC: Office of Aerospace Medicine, Civil Aerospace Medical Institute; 2008.
- [Google Scholar]
- Analysis of drugs of abuse by gas chromatography-mass spectrometry (GC-MS) Methods Mol Biol. 2018;1810:29-42.
- [CrossRef] [PubMed] [Google Scholar]
- Simultaneous detection and quantification of amphetamines, diazepam and its metabolites, cocaine and its metabolites, and opiates in hair by LC-ESI-MS-MS using a single extraction method. J Anal Toxicol. 2008;32:457-69.
- [CrossRef] [PubMed] [Google Scholar]
- Development and validation of a single LC-MS/MS assay following SPE for simultaneous hair analysis of amphetamines, opiates, cocaine and metabolites. Forensic Sci Int. 2014;234:132-8.
- [CrossRef] [PubMed] [Google Scholar]






