Translate this page into:
Hyperbaric oxygen therapy as a treatment option for intractable hemorrhagic cystitis in a pediatric patient: A case report

*Corresponding author: Deepan Rai, Institute of Aerospace Medicine, Bengaluru, Karnataka, India. raideepan82@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Rai D, Sarkar R, Ningaiah M. Hyperbaric oxygen therapy as a treatment option for intractable hemorrhagic cystitis in a pediatric patient: A case report. Indian J Aerosp Med 2024;68:20-4. doi: 10.25259/IJASM_6_2023
Abstract
Hyperbaric oxygen therapy (HBOT) is gaining importance as a treatment modality for hemorrhagic cystitis (HC). At pressures of more than 1 atmospheric absolute, administering 100% oxygen initiates neovascularization and mucosal layer formation and reduces edema. HC is a pathological condition manifested with recurrent hematuria, urinary urgency, and suprapubic pain. HC can be caused by radiation exposure, chemotherapeutic drugs, and viral and bacterial infections. HC is a common sequela after stem cell transplant (SCT). Vascular injury and inflammation of bladder mucosa with the use of certain immunosuppressants and post-SCT is a known condition that leads to HC. HC is classified into two types based on the time of onset of the disease following chemotherapy/radiotherapy: Early and late onset of HC, which appears within 48–72 h and 2–3 months, respectively. HC is one of the approved indications for the administration of HBOT from the Undersea and Hyperbaric Medicine Society. There is scanty evidence of HBOT being effective in pediatric cases of hemorrhagic cystitis; however, the effectiveness of the therapy in a pediatric patient on opioid analgesic and active bladder irrigation was debatable. A case of a 6-year-old male child treated with a haploidentical SCT for X-linked adrenoleukodystrophy, was followed up with post-transplant cyclophosphamide. He developed intractable Grade III HC 2 weeks after transplant. The decision-making in assessing the fitness of this child to undergo therapy and the results achieved are discussed in detail in this paper.
Keywords
Hemorrhagic cystitis
Stem cell transplant
Hyperbaric oxygen therapy
X-linked adrenoleukodystrophy
Cyclophosphamide
INTRODUCTION
Hyperbaric oxygen therapy (HBOT) is a treatment modality in which a patient is administered 100% oxygen intermittently inside a hyperbaric chamber that is pressurized higher than 1 atmospheric absolute (ATA).[1] The course of hyperbaric oxygen (HBO2) has progressed by leaps and bounds over the past 3 centuries.
In 1917, Dräger found therapeutic use of oxygen when administered under high pressure. He also invented a system for treating accidents in divers. Nonetheless, his system never went into mass production and utilization.
Jain Kewal, in 1937, used HBOT for the 1st time for the treatment of decompression sickness.[2] Thereafter, HBOT has been used as a treatment modality for various diseases. The Undersea and Hyperbaric Medicine (HM) Society has specified 14 indications of HBOT.[1] In some conditions, it is a primary modality of treatment, and in others, it is an adjunct to the primary therapy.
Hemorrhagic cystitis (HC) is one such indication in which HBOT is useful. HC is a pathological condition manifested with recurrent hematuria, urinary urgency, and suprapubic pain. The inflammation of the mucosal layer of the urinary bladder, followed by mucosal rupture, leads to hemorrhagic cystitis in patients who have undergone chemotherapy, radiotherapy, and bacterial and viral infections. In chemotherapy-induced hemorrhagic cystitis, urotoxicity is caused by the renal excretion of acrolein metabolites of oxazaphosphorine alkylated drugs.[2] Stem cell transplant (SCT) is an accepted treatment modality for malignancy. In the state of immunosuppression, post-SCT makes an individual susceptible to opportunistic infections such as the BK virus (BKV), which, in turn, causes HC. BKV is attributed to the late onset of HC in immunosuppressed, immunocompromised individuals.[3] In SCT, the incidence of HC is revealed to be increased by 50% or more.[2] The treatment of cases of HC with HBOT following BKV infection has shown promising results.[4] In adults, where radiation is the most common cause of HC, immediate and late effects of stem cell or bone marrow transplant for various malignant and benign conditions are the most common cause in children.[4] It is a proven fact that HBOT has minimal risk, and it is effective in treating pediatric patients with HC. However, it is not being used commonly due to the cost and limited availability of HBOT facilities.[4]
The present case is a pediatric case with an intractable HC post-SCT who was treated with HBOT at the Institute of Aerospace Medicine (IAM), Bengaluru. The HBOT Chamber, or the Rapid Recompression Chamber at IAM, Indian Air Force, is a multiplace type that was installed as a safety requirement following the installation of an explosive decompression chamber for treatment of accidental cases of decompression sickness and was manufactured by Kasco Industries, Pune, India.
CASE REPORT
The case was a 6-year-old male child diagnosed with X-linked adrenoleukodystrophy, admitted and treated in a civil hospital with haploidentical SCT with post-transplant cyclophosphamide. He developed viral pneumonia on day 0 of hospital admission; on evaluation, he was found to be respiratory syncytial virus positive as well. He was managed with ribavirin, intravenous antibiotics, and oxygen support.
The child developed HC 2 weeks after transplant, which was managed conservatively. Investigation revealed BKV copies of 509 and adenovirus copies of 25 million, and his coagulation profile was normal. He was treated with an injection cidofovir at the rate of 3 mg/kg weekly; however, symptoms of HC persisted. Foley’s catheterization with continuous irrigation was started on day 37 of admission. USG abdomen revealed a clot size of 30 cc in the bladder, which increased to 108 cc in subsequent weeks (on day 48).
Due to severe pain, increasing clot size, and peri-catheter leakage, supra-pubic catheterization with cystoscopic hematoma evacuation was done on day 49. Bladder irrigation was continued with a suprapubic catheter. Given continuous pain with peri-catheter leakage, ureteric diversion with cystoscopic hematoma evacuation was carried out on day 61. After one month, suprapubic catheter and bilateral ureteric catheter removal were done.
After 11 weeks of onset of HC, he was referred to the IAM, Department of High Altitude Physiology and HM with severe grade III HC for HBOT. He was being treated with continuous bladder irrigation and injectable opioid analgesics through infusion. He was evaluated at IAM before subjecting him to HBOT. The Pulmonary Function Test was performed following the latest American Thoracic Society guidelines, which revealed a restrictive pattern. This finding was considered to be due to his poor general condition. As all other radiological, biochemical, and hematological markers were normal, the child was considered fit to undergo HBOT.
The opioid-based analgesic infusion was stopped due to potential respiratory depression, which could have made HBOT inefficient.
The irrigation bottle was half emptied before subjecting the patient to recompression to accommodate the pressure effects on the container. The patient was accompanied by his father and a medical assistant inside the hyperbaric chamber [Figure 1].
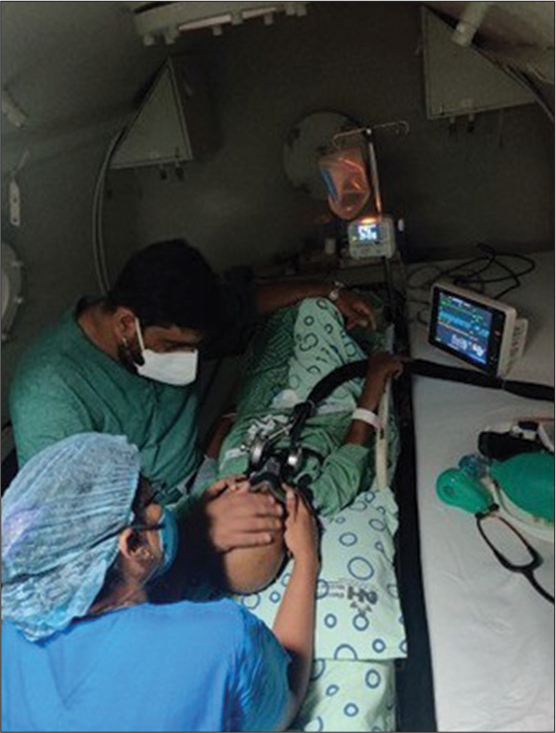
- Case being treated in hyperbaric chamber.
The profile used was a slow descent to a depth of 50 ft or 2.5 ATA at the rate of 2 ft/min. 100% oxygen was initiated at the simulated depth of 50 ft and was continued for 90 min.
An oxygen break of 5 min was provided every 25 min [Figure 2]. He was accompanied by a medical officer in the initial sessions of HBOT and later by a medical assistant. He was closely monitored inside the chamber and from the control station as well. In the initial few sessions, the patient experienced excruciating pain in the lower abdomen, for which reassurance and an injection of tramadol were administered. The intensity of pain reduced with continuing sessions. The visible examination of urine collected in the urine bag showed a reduction in red color after seven sessions of HBOT, which is evident in Figures 3 and 4 below. A total of 18 sessions were given on successive days; however, a break of a day due to fever on 1st and 3rd week of HBOT was given as advised by his pediatrician. After the completion of 18 sessions, USG revealed a complete resolution of hematoma of the bladder with no active bleeding. On follow-up after 5 months, the child did not have any recurrence of symptoms; he could carry out his daily routine on his own and resumed going to school.
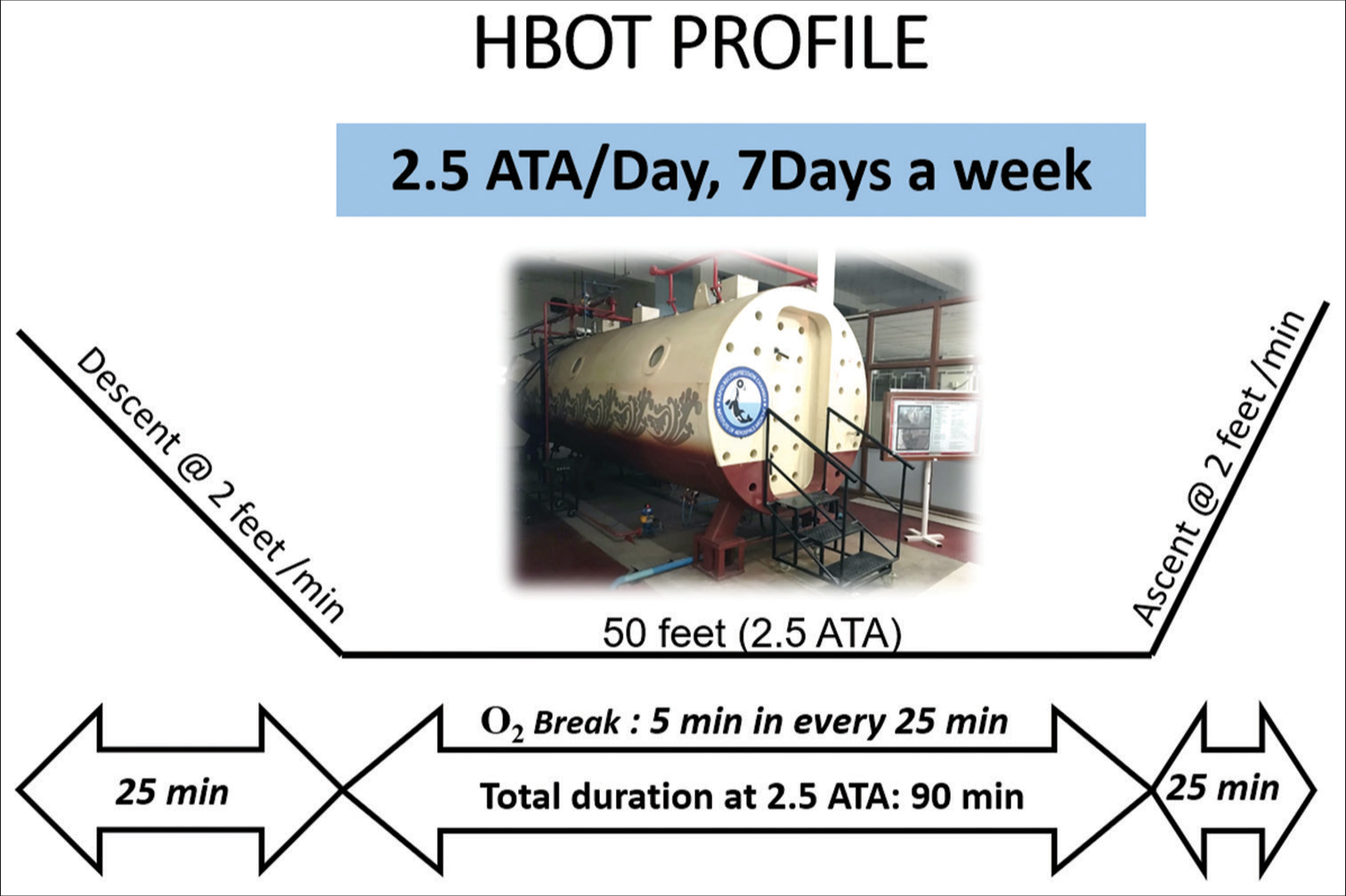
- Hyperbaric oxygen therapy (HBOT) protocol administered for the patient, ATA: Atmospheric absolute.
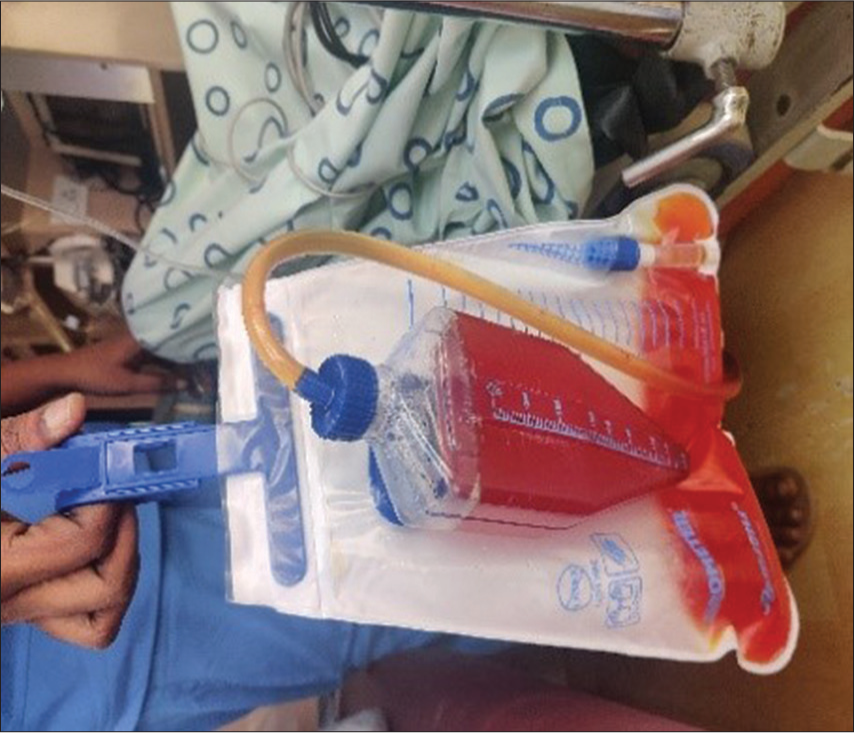
- Frank hematuria before hyperbaric oxygen therapy.

- Appearance of urine after hyperbaric oxygen therapy sessions.
DISCUSSION
HC is well documented and common in post-SCT cases primarily due to vascular injury and inflammation of the bladder mucosa.[5] HBOT is being used in the treatment of HC of various etiologies. HC can be broadly classified into two types based on the duration of onset.[5]
Early-onset HC: Manifest in 48–72 h post chemotherapy
Late-onset HC: Manifest in 2–3 months post HSCT.
Brugieres et al. defined the criteria of grade of HC.[6]
Grade I: Gross hematuria and thrombocytopenia
Grade II: Gross hematuria and clots
Grade III: Gross hematuria, clots, and urethral obstruction.
The role of HBOT in children with hemorrhagic cystitis is not well defined in the literature. A case of 18-month-old patient with stage 2 embryonal rhabdomyosarcoma of the bladder was treated with cyclophosphamide and pelvic radiation. Despite the administration of 2-mercaptoethane sodium sulfonate (mesna), she developed gross hematuria and clot formation after a month. She was hospitalized multiple times, and she even required a blood transfusion. Investigation revealed bladder mucosa hemorrhagic and inflamed. She was managed with bladder irrigation followed by clot evacuation. Despite the treatment, she remained symptomatic. She was referred for HBOT and underwent ten sessions of HBOT, each of 60 minutes duration at 2.0 ATA. Following the treatment, her symptoms resolved completely. There was no recurrence of symptoms during follow-up.[7]
A study conducted by Cesaro et al. has revealed that the cure rate of HC is 78.5%, with HBOT in patients not responding to other medical treatments.[5] Another study conducted by Lopez et al. has concluded that HBOT in HC has good prognosis and also helps preventing potential sequelae. Even severe forms HC can be completely treated with HBOT.[3]
HBOT works by multiple mechanisms in Hemorrhagic cystitis. A study conducted by Monaca et al., where patients were subjected to 90 min of HBOT intermittently, revealed decreased activated partial thromboplastin time and increased leukocyte count.[8] It indicates the activation of platelets following HBOT through thrombin and arachidonic acid pathways.[2] It is also found that HBOT increases the elasticity of erythrocytes.[2] This enhances the flow of red blood cells through the capillaries and improves tissue oxygenation.
Given the phenomenal result of the treatment, it is prudent to consider HBOT in such cases in the early stage of the disease to avoid complications and patient distress.
CONCLUSION
HBOT is an efficacious and promising modality for the treatment of a pediatric patient with hemorrhagic cystitis in whom conventional modalities of management have failed. It is a viable option in refractory HC. Conventional treatment for refractory HC is usually invasive and may cause injury to the bladder. However, HBOT is non-invasive, and it heals the tissue permanently by reducing inflammation and inducing neoangiogenesis while keeping the bladder function intact. Therefore, HBO2 should be considered early when HC does not respond to conventional management.
Ethical approval
Institutional review board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript, and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Undersea and hyperbaric medical society: Hyperbaric oxygen therapy indications. 2019. Best Publishing Company. (14th ed). Available from: https://www.uhms.org/images/UHMS-Reference-Material.pdf [Last accessed on 2023 Jun 14]
- [Google Scholar]
- Hyperbaric oxygen therapy of an adolescent stem cell transplantation recipient with hemorrhagic cystitis and BK virus. Case Rep Pulmonol. 2020;2020:3465412.
- [CrossRef] [PubMed] [Google Scholar]
- Canadian urological association best practice report: Pediatric hemorrhagic cystitis. Can Urol Assoc J. 2019;13:E325-34.
- [CrossRef] [PubMed] [Google Scholar]
- Incidence and treatment of hemorrhagic cystitis in children given hematopoietic stem cell transplantation: A survey from the Italian association of pediatric hematology oncology-bone marrow transplantation group. Bone Marrow Transplant. 2003;32:925-31.
- [CrossRef] [PubMed] [Google Scholar]
- Hemorrhagic cystitis following high-dose chemotherapy and bone marrow transplantation in children with malignancies: Incidence, clinical course, and outcome. J Clin Oncol. 1989;7:194-9.
- [CrossRef] [PubMed] [Google Scholar]
- Hyperbaric oxygen therapy for pediatric hemorrhagic cystitis. J Urol. 1999;161:1596-7.
- [CrossRef] [Google Scholar]
- Assessment of hemostaseologic alterations induced by hyperbaric oxygen therapy using point-of-care analyzers. Undersea Hyperb Med. 2014;41:17-26.
- [Google Scholar]






